| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 1: Harry D Bear, MD, PhD

| Edited comments by Harry D Bear, MD, PhD |
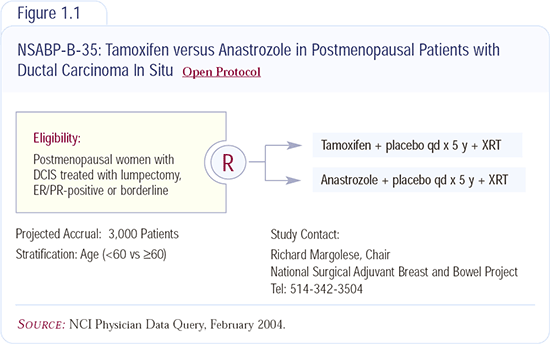
NSABP-B-35: Tamoxifen versus anastrozole in patients with DCIS
This study builds on the series of DCIS trials: NSABP-B-17, which proved the value of radiation therapy, and NSABP-B-24, which demonstrated that the addition of tamoxifen improves recurrence rates. NSABP-B-35 builds on NSABP-B-24 and the ATAC trial, which demonstrated an advantage for anastrozole versus tamoxifen for invasive breast cancer.
The side-effect profile of anastrozole seems to be a little more acceptable than that of tamoxifen, particularly related to hot flashes. Patients also have concerns about uterine cancer, and anastrozole does not have that problem. However, there are tradeoffs. Anastrozole has the issue of osteoporosis, but that’s the point of a randomized trial — to compare the two. I don’t currently recommend anastrozole for DCIS outside of a trial. The NSABP-B-35 study is requiring ER testing because it appears that patients who have ER-negative DCIS probably don’t benefit from taking tamoxifen.
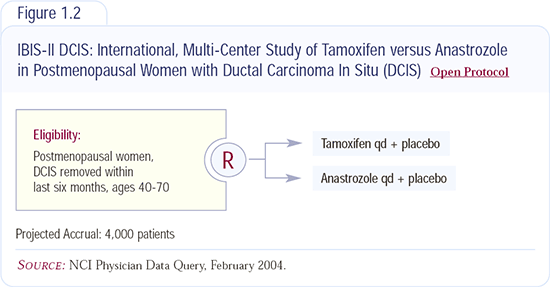
Role of radiation therapy for DCIS
Surgeons and patients alike question whether radiation therapy is necessary for all patients with DCIS. The NSABP trials demonstrated radiation therapy conferred benefit in every subset of patients who had DCIS. On the other hand, Dr Silverstein’s extensive experience suggests that some patients — particularly those with a wide margin resection — do not derive much benefit from radiation. In my practice I find that the cosmetic results of performing a limited resection and adding radiation therapy are superior to the results of a large excision with a wide margin.
There are individual patients in whom I might avoid radiotherapy, such as patients with a very small focus of DCIS or those who had a core biopsy of DCIS with cores from multiple sites. I’d do a definitive excision and, if there’s no residual cancer in the breast, those patients probably will have acceptable outcomes without radiation therapy — particularly patients over 70 years old.
Another development that may apply to DCIS is the partial breast radiotherapy trial. That’s a very exciting way to further improve the cosmetic result and decrease the concerns about going through six weeks of whole breast radiotherapy, which is, at best, inconvenient.
DCIS and sentinel lymph node biopsy (SLNB)
There’s been a trend of performing SLNB in patients with DCIS, which is absurd for the majority of patients. However, in select patients, I’ll consider doing a sentinel node biopsy. For example, in patients with a very aggressive-looking DCIS and microinvasion, when I do a definitive lumpectomy I know there’s a chance the pathologist will find definitive invasion, and I can potentially avoid another operation by performing SLNB. I also do sentinel node biopsy in patients undergoing a mastectomy because if the pathologist finds invasive cancer in the definitive breast specimen, it is too late to perform a sentinel node biopsy because the breast has already been removed.
Neoadjuvant therapy and SLNB
Until recently, I’ve recommended most patients receiving neoadjuvant therapy have an axillary node dissection because we haven’t known the accuracy rate of sentinel node biopsy in that setting.
Some have advocated performing sentinel node biopsy before chemotherapy, and have shown that it works. I think it’s probably more useful to the patient to do it afterwards. We now have some of the data from NSABP-B-27, a trial in which 300 to 400 patients had sentinel node biopsies at the time of surgery followed by axillary node dissection. It was not designed as a part of the trial. It was simply coincidental that a lot of surgeons were performing sentinel node biopsies, perhaps to gain more experience with the technique. The false-negative rate was approximately 11 percent, which is similar to the rates in the multicenter trial performed by David Krag. The technique was not standardized and, again, it’s likely some of the surgeons were still early in the learning curve.
Performing the sentinel node biopsy after neoadjuvant chemotherapy is preferable because it allows axillary node dissection to be avoided in the maximum number of patients. The sentinel node should be negative in patients in whom it was initially negative, and it will be negative in patients whose nodes have been sterilized by the chemotherapy. Regardless of whether those nodes were always negative or have been converted to negative, the prognostic significance is the same, or even greater, than it would have been from knowing the nodal status up front.
The problem with knowing the nodal status before treatment is that you don’t know what to do with that information. If the patient was node-positive prior to systemic therapy, does that automatically mean the patient should have an axillary node dissection? I guess the answer is “yes,” but if you do the axillary node dissections after chemotherapy you’re going to perform a lot of axillary node dissections and find no tumor.
Proposed NSABP-B-27 neoadjuvant replacement trial
The NSABP-B-27 replacement study represents a paradigm shift in how we perform neoadjuvant trials, particularly with the advent of molecular markers and gene expression signatures as predictors of response. We felt it would be wasteful to put thousands of patients into a two-arm study and wait five to 10 years for the survival data to mature.
We will use the neoadjuvant studies as a discovery platform to compare multiple regimens in a very rapid-fire way. By using pathologic complete response as the primary endpoint and doing molecular marker analysis and gene expression profiles on all of the patients prior to, in the middle of and after chemotherapy, we hope to develop markers that can be used to predict responses to certain drugs.
We envision that the B-27 replacement trial will evaluate AC plus a taxane or several taxane combinations to predict pathologic complete response. We’ll also switch the order of treatment so that we can determine whether genetic profiles can predict response to either anthracyclines or taxanes.
Proposed NSABP trial evaluating chemotherapy for local recurrence
Local recurrence in patients who have undergone breast conservation surgery, or a chest wall or regional recurrence in patients who’ve had a mastectomy, is a signal that the patient is likely to develop metastatic disease in the near future.
We don’t know whether giving chemotherapy in that setting will alter the outcome, particularly in patients who previously received adjuvant chemotherapy. We’ve decided to join a large international trial that will examine that question, and rather than specifying a specific chemotherapy regimen, we’ll leave the chemotherapy up to the individual investigator.
Select publications
|
Dr Bear is the Chairman of the Division of Surgical Oncology, Professor of Microbiology and Immunology, Walter Lawrence Jr, Distinguished Professor in Oncology at the Massey Cancer Center, Virginia Commonwealth University School of Medicine in Richmond, Virginia. |
|
