|
You are here: Home: BCU Surgeons 2004 Vol 3 Issue 1: Anthony Howell, MD, MSc, FRCP

| Edited comments by Anthony Howell, MD, MSc, FRCP |
ATAC analysis of response based on progesterone receptor assays
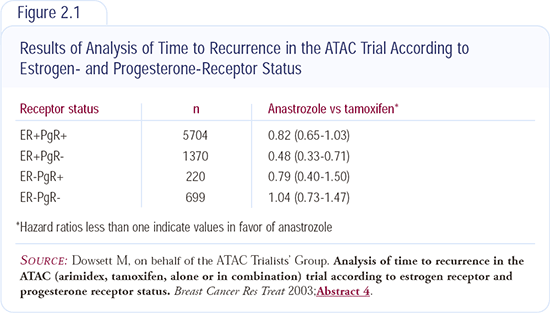
The analysis of recurrence according to estrogen and progesterone receptor status was the first translational research component of the ATAC trial to be reported. The data indicate patients with ER-positive and PR-negative tumors — approximately 20 percent of postmenopausal ER-positive patients with breast cancer — have a 50 percent reduction in the hazard for recurrence compared to tamoxifen, whereas those with ER/PR-positive tumors have about a 20 percent reduction in the hazard ratio (Figure 2.1).
We need to be cautious because there are early data and it’s the first time this pattern has been reported. Biologically, it makes sense, because ER-positive/PR-negative tumors tend to be HER2-positive in other trials with which we’ve been involved. Additionally, in the letrozole preoperative trial and the IMPACT neoadjuvant anastrozole trial, patients with ER-positive, HER2-positive disease responded better to aromatase inhibitors than to tamoxifen. We haven’t yet evaluated HER2 status in the ATAC trial.
Side effects and toxicities of anastrozole versus tamoxifen
The major difference between the two drugs is gynecologic — less bleeding and less endometrial cancer with anastrozole, and fewer strokes and deep vein thromboses. The down side of anastrozole is aching in the joints, vaginal dryness and effects on bone.
Aromatase inhibitors as initial therapy and sequence after tamoxifen
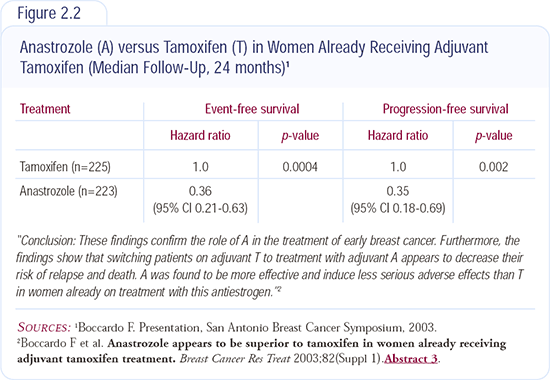
Increasingly, more data are emerging to support the superiority of aromatase inhibitors over tamoxifen. The NCIC-MA17 trial demonstrated the value of letrozole after five years of tamoxifen, and the Italian trial (Figure 2.2) just reported at San Antonio indicated that the switch from tamoxifen to anastrozole at two or three years results in a disease-free survival advantage and nearly results in a statistically significant survival advantage (p = 0.06).
I believe that if you’re going to use an aromatase inhibitor, it is most appropriate to use it up front. The data in this setting are with anastrozole, so if I am going to use an aromatase inhibitor up front, I use anastrozole. The data for switching from tamoxifen at two to three years are with anastrozole, so I use anastrozole in that setting. After five years of tamoxifen, the data are with letrozole, so I use letrozole in those patients. Good clinical scientists treat patients according to the data. Figure 2.2
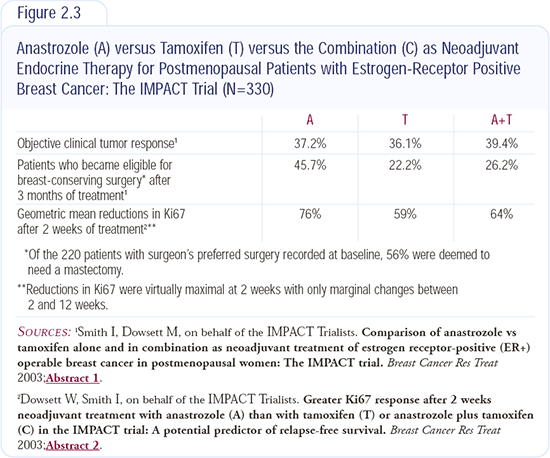
IMPACT neoadjuvant trial: Anastrozole versus tamoxifen versus the combination
The IMPACT trial (Figure 2.3) can be thought of as preoperative ATAC, with treatment given for three months. Response rates were similar in all three arms —approximately 30 percent by calipers — but breast conservation rates were significantly higher with anastrozole. In the biological study reported, anastrozole resulted in approximately a 20 percent reduction in the proliferation index Ki67, compared to either tamoxifen or the combination, which was similar to the ATAC trial results. Additionally, the response rate was higher in patients with HER2-positive disease, which mirrors Matt Ellis’ data with letrozole.
Select publications
|
| Dr Howell is a Professor of Medical Oncology at the University of Manchester in Manchester, England. |
|
|
|
|
