| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 1: Edith A Perez, MD
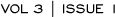

| Edited comments by Edith A Perez, MD |
Concordance between local and central laboratory HER2 testing
We published data on the first 119 specimens submitted to our adjuvant trial. We were surprised to find poor concordance between community and central laboratory testing, in terms of both HER2 protein expression and gene amplification.
In the same issue of the Journal of the National Cancer Institute, the NSABP published a paper evaluating specimens from their adjuvant trial (Figure 4.1). Amazingly, their data were almost identical to ours in terms of the discordance rate; however, they found the discordance rate to be much lower when experienced or certified laboratories for HER2 testing were used. Physicians in the community need to send specimens to experienced laboratories.
Algorithm for HER2 testing: IHC versus FISH
We recommend an algorithm that starts with immunohistochemistry, because it is an easier, less expensive test to do. If the tumor is IHC 0, 1+ or 3+, no further testing is necessary. If the tumor is IHC 2+, reflex FISH testing is recommended (Figure 4.2). At our facility, the pathologists automatically perform the FISH analysis.

We believe perhaps it’s not a good idea to do FISH testing for every tumor, because the majority will be negative. Should we test 100 percent of tumors to find 25 percent positive, or should we use immunohistochemistry to guide us in terms of FISH testing? The latter approach will save money and time.
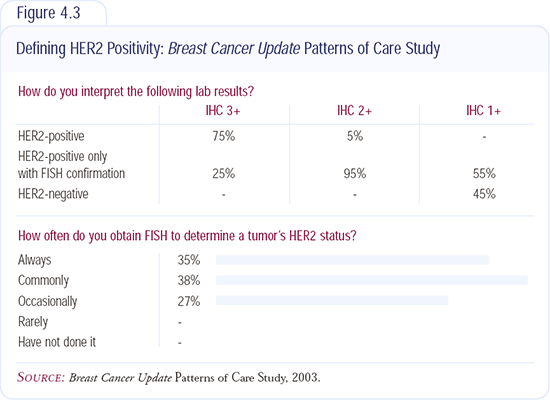
Routine HER2 testing on breast cancer specimens
Our approach is to test all primary breast cancers for HER2 (Figure 4.3). This is part of the standard evaluation of all new invasive breast cancers diagnosed at the Mayo Clinic in Jacksonville.
Knowing a tumor’s HER2 status helps in three ways. First, it may assist in determination of prognosis, especially in patients with node-positive breast cancer. Second, it may give us an idea of the potential benefit of anthracycline-versus non-anthracycline-based chemotherapy in the adjuvant setting. Finally, knowing the HER2 status allows us to identify patients who may be eligible for adjuvant trastuzumab protocols such as N-9831.
I do not know what fraction of breast tumors are being tested for HER2 nationwide, but some institutions only test tumors from patients who are node-positive. I believe this is changing as education has improved and there is increased awareness of the potential value of HER2 testing in the determination of prognosis and decisions for therapy.
The discordance rate becomes much lower by using experienced or certified laboratories for HER2 testing, which is good for clinical care because we need to remember that HER2 testing is not only being done for patients potentially eligible for clinical trials, but also for general clinical practice.
Intergroup adjuvant trial evaluating trastuzmab plus chemotherapy
This trial builds on several issues, including the relative importance of anthracyclines in patients with HER2-positive breast cancer, and the value of adjuvant taxanes. Patient randomly assigned to trastuzumab receive it for a year. I believe adjuvant trastuzumab currently should only be used in a clinical trial setting. Clinicians who use this therapy off protocol are essentially shooting in the dark, because we don’t understand for how long this therapy should be given, what schedule should be used in combination with chemotherapy, and the potential risks or benefits patients may derive from such treatment. There are several major clinical protocols available, and I hope that every woman diagnosed with HER2-positive breast cancer asks her physician about participation in a clinical trial that will help answer those questions.
Select publications
|
| Dr Perez is Professor of Medicine at the Mayo Medical School, Director of the Cancer Clinical Study Unit, Director of the Breast Cancer Program, Division of Hematology and Oncology, at the Mayo Clinic in Jacksonville, Florida. |
|
