| You are here: Home: BCU 2|2004: Julie R Gralow, MD

Edited comments by
Julie R Gralow, MD |
Planned SWOG trial evaluating the use of adjuvant bisphosphonates
Our Intergroup trial will compare clodronate to risedronate, a more potent oral bisphosphonate, and to zoledronate, an intravenous bisphospho-nate, which would be considered our standard of care in the metastatic setting. Our primary endpoints will be prevention of bone metastases and disease-free and overall survival. Clodronate and risedronate will be administered daily for three years, and zoledronate will be given monthly for the first six months, and then on an every three-month schedule for the remaining two and a half years.
We will accrue approximately 6,000 patients and eligibility is pretty basic. We want to enroll patients who are receiving adjuvant treatment. Patients enrolled can receive any type of hormonal therapy or chemotherapy. We're also allowing co-enrollment in other clinical trials, as long as bone density isn't a major endpoint. Any patient at a low enough risk that they would not receive adjuvant systemic therapy will be excluded from the study.
There is some preclinical data suggesting the aminobisphosphonates risedronate and zoledronate may have some direct antitumor effect. My hypothesis is that these more potent agents have some slightly different mechanisms than clodronate and will be more effective. There is a reasonable chance that the bisphosphonates can impact survival and decrease bone metastases.
That being said, I'm not sure how bisphosphonates will be used, especially in patients at low risk, because I believe they will cause some toxicity. In the future, we may select a group of patients who are most likely to develop bone metastases and collect tumor blocks and serum for markers of bone turnover. One somewhat controversial hypothesis in this regard relates to the parathyroid hormone-related peptide (PTHrP) receptor. There are some measurable tumor characteristics that may predict for tumors more likely to metastasize to the bone.
We will also have a small substudy population - about 20 patients in each arm - in whom we will perform bone biopsies so we can evaluate bone quality by labeling, compression and nuclear medicine techniques. These studies should allow us to truly see what is happening to bone quality.
Rationale for evaluating bisphosphonates in the adjuvant setting
Among breast cancer patients who develop metastases, 70 to 80 percent will have bone metastases. In 40 to 50 percent it will be the first site of metastases. Before breast cancer tumor cells are evident as bone metastases, they can secrete a variety of cytokines that stimulate osteoclasts. They can also impact osteoblasts, macrophages and other cells. In stimulating the osteoclasts, the cell is encouraging bone breakdown and, in turn, the bone microenvironment -osteoclasts, osteoblasts and macrophages - will make cytokines that stimulate the breast tumor cells, so breast cancer cells and the osteoclasts have an intimate relationship and can feed each other. We know from the metastatic setting that bisphosphonates can inhibit osteoclasts and prevent or delay bone breakdown.
Prior clinical trials of adjuvant bisphosphonates
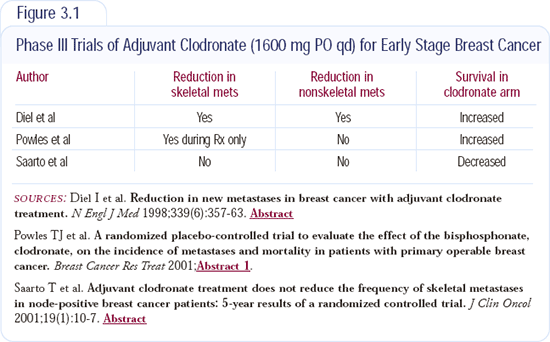
Three clinical trials evaluating adjuvant bisphosphonates have been reported from Europe (Figure 3.1). The first trial, reported by Dr Diel from Germany, selected patients who had known positive bone marrow aspirates but no other metastatic disease. Approximately 300 patients were randomly assigned to clo-dronate or placebo. Those receiving clodronate had reduced bone metastases and improved survival.
A study from Scandinavia demonstrated virtually the opposite findings. Three years of adjuvant clodronate resulted in a worse survival compared to placebo. That study resulted in no difference in the incidence of bone metastases.
Trevor Powles presented data from a United Kingdom-led trial with about 1,000 unselected patients receiving adjuvant therapy. That study reported a small but significant survival benefit. During the two years patients received clodronate on study, fewer bone metastases were observed. As soon as patients discontinued clodronate, the bone metastases seemed to even out in the two groups.
The data are not conclusive. We have one negative trial, and the largest trial demonstrated a small but real survival benefit. Recent letters to the editors of journals suggest that long-term, high-dose bisphosphonates may potentially cause some problems. While we need to investigate the protective effect of bisphos-phonates, we also need to be certain we aren't inducing toxicity in patients.
Bisphosphonate-associated osteonecrosis
Bisphosphonates increase bone density, but they also remain in the bone for years and years. That is a potential problem because they may impair bone quality. Although bones treated with bisphosphonates may appear to be denser on a Dexascan, they may not be scaffolded and structured as well in terms of their lay-down of calcium and phosphate.
Two recent letters to the editor in the Journal of Oral Maxillofacial Surgery and the Journal of Clinical Oncology document patients who had dental extractions that failed to heal. The patients developed osteonecrosis and needed to be treated with antibiotics. This may be a phenomenon peculiar to the jaw and may not have anything to do with fractures or surgery anywhere else, but we need to look at this.
ABCSG-12: LHRH agonist with tamoxifen or anastrozole with or without zoledronate
The Austrian Breast Cancer Study Group (ABCSG) trial 12 demonstrated increased bone density from zoledronate at six months and one year among patients treated with an LHRH agonist plus tamoxifen or anastrozole. We need to follow that study because these were early data from only about 100 patients, and it's a much larger trial than that.
I'm regularly asked, "Should I automatically administer a bisphosphonate when starting an aromatase inhibitor?" I would prefer to monitor bone density. There are patients who won't need a bisphosphonate at all. In our update of the MA17 trial of letrozole versus placebo after five years of tamoxifen, we really don't have substantial numbers of fractures. Currently, there is a one percent fracture rate in the study. Most of our patients aren't going to run into big trouble quickly, so you can do a baseline Dexascan, monitor patients and institute bisphosphonates at an appropriate time based on the WHO criteria for osteoporosis and osteopenia.
Fulvestrant in combination with a dual tyrosine kinase inhibitor
We're going to perform a Phase II study combining fulvestrant with GW572016, a dual HER1 and HER2 tyrosine kinase inhibitor, in patients with metastatic disease. There were several abstracts presented in San Antonio suggesting that HER2-positive, ER-positive tumors have resistance to tamoxifen, fulvestrant and the aromatase inhibitors. Targeting HER1 - the epidermal growth factor receptor - and HER2 might allow us to overcome resistance to endocrine therapy.
Fulvestrant for metastatic breast cancer
Fulvestrant is an active agent, but I'm not sure we're utilizing the best dose. I'd be interested in whether it is feasible to utilize a loading dose or more frequent administration initially to get the levels up.
Many of my patients have received adjuvant tamoxifen, so I typically use first-line aromatase inhibitors off-study and administer fulvestrant upon progression. Subsequently, we may readminister tamoxifen, utilize progestin agents or try another aromatase inhibitor. Many of our patients with hormone receptor-positive metastatic disease can be maintained on hormonal therapies for several years before we have to treat them with chemotherapy.
SWOG trial S0221: Dose-dense versus metronomic scheduling of chemotherapy
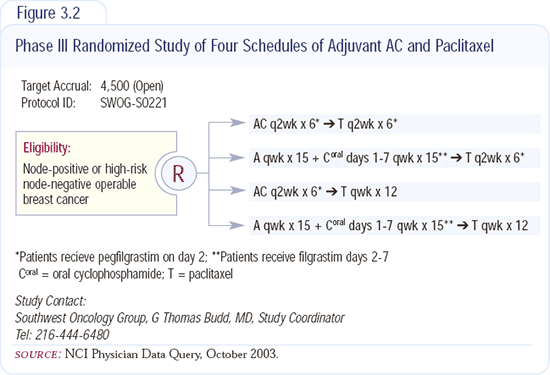
SWOG has just opened the new Intergroup adjuvant trial S0221 (Figure 3.2). It is testing the dose-dense concept of every two-week AC and every two-week paclitaxel versus a metronomic dosing schedule. Doxorubicin is administered weekly and oral cyclophosphamide is given daily.
It's a two-by-two design, so we have two different ways of administering the anthracycline and cyclophosphamide and two different ways of administering the paclitaxel. We're comparing paclitaxel every two weeks plus growth factor support to a weekly schedule of the drug.
Data indicate that oral cyclophosphamide, in both the metastatic and adjuvant combination regimens, may be the better way of administering the agent, and that the weekly doxorubicin has more myelotoxicity and bone marrow toxicity but less cardiotoxicity and swings in fatigue. Over time, any chemotherapy adds up, but because you're giving smaller doses more frequently, there are fewer ups and downs.
We tested this regimen in the neoadjuvant setting in SWOG-9625, led by Dr Georgiana Ellis. In that study, we just gave the anthracycline and cyclophosphamide without the taxane. The primary endpoint was pathologic complete response rate.
We only enrolled patients with T3 and T4 tumors, and approximately one-half had inflammatory breast cancer; this was a pretty high-risk group. We administered 16 weeks of therapy with this regimen and growth factor support. It was tolerable in a multi-institution setting and resulted in a pathologic complete response rate of 25 percent.
That pathologic complete response rate occurred with an anthracycline and an alkylating agent and without a taxane. Those results are comparable to the results of AC followed by docetaxel in NSABP-B-27. SWOG-9625 wasn't a randomized trial, but with such a good pathologic complete response rate, we're very interested in comparing it to what now probably is considered to be the standard of care in many places - the every two-week, dose-dense schedule.
Nonprotocol adjuvant chemotherapy
Currently, the weight of the evidence probably supports the dose-dense AC/paclitaxel regimen. TAC may be as efficacious as the dose-dense regimen. Data from the TAC/FAC adjuvant study have been updated and demonstrate a survival benefit for replacing 5-FU with the taxane. AC in combination with docetaxel in a sequential manner is probably tolerated better and may be just as efficacious, but again, we only have surgery data from NSABP-B-27, not long-term results.
The Aberdeen trial - CVAP, and if responding to four cycles, randomized to four more cycles of CVAP versus docetaxel - was recently updated. This small, 160-patient study had significantly better pathologic complete response rates - even in responders to an anthracycline - than switching to docetaxel. It's impressive that they were able to demonstrate a statistically significant survival advantage with such small numbers.
We don't have a head-to-head comparison between docetaxel and paclitaxel in the adjuvant setting. The recent update of the TAX-311 study demonstrated that in metastatic disease, docetaxel every three weeks was superior to paclitaxel every three weeks. That's the reason we're looking at different ways of giving paclitaxel - weekly versus every two weeks with growth factors.
Adjuvant clinical trials of chemotherapy in lower-risk patients
We're participating in the Intergroup trial, CALGB-40101, led by Larry Shulman, which asks, "Is AC for six cycles better than four cycles?" This study also attempts to determine whether anthracyclines are necessary or whether they could be replaced with a taxane to avoid the cardiotoxicity. It's a four-arm study - AC for four cycles or six cycles every two weeks, or paclitaxel administered every two weeks for four versus six cycles. After the dose-density data were presented they decreased the timing from every three weeks to every two weeks, all with growth factor support.
In our older patients, Hyman Muss is leading CALGB-49907, evaluating whether we can administer capecitabine as a single agent in the adjuvant setting. Capecitabine may not result in the hair loss associated with other regimens, and it may be less toxic. These studies in more fragile patients and patients at lower risk are asking whether we can avoid anthracycline-based regimens entirely with equivalent results and less toxicity.
CALGB trial 49907 of adjuvant chemotherapy in the elderly
CALGB-49907 randomly assigns patients to conventional chemotherapy, AC or CMF versus capecitabine. There were many discussions early on regarding the dose of capecitabine. Few physicians are starting at the FDA-approved dose of 2,500 mg/m2 daily in two divided doses - two weeks on, one week off. Thus, the trial was started at 2,000 mg/m2 daily in two divided doses.
We had to halt the study after two deaths occurred in the capecitabine arm. One death happened in the fifth or sixth cycle in a patient who was somewhat removed from the medical system due to family problems. She continued taking capecitabine despite GI toxicity and subsequently died. The other death was clearly a classic case of dihydropyrimidine dehydrogenase (DPD) deficiency. We struggled with that since we really don't know how to test for it in a reliable way.
We know that DPD deficiency will occasionally occur with 5-FU or a 5-FU pro-drug like capecitabine, and its estimated incidence is probably about one to two percent. However, we cannot have deaths occurring in people who may already be cured, so we put the capecitabine arm on hold while we tried to figure out how to identify those rare cases of DPD deficiency and how we could better monitor our patients.
We have recently reopened the capecitabine arm with a mandated medical visit within the first week. During this visit, blood counts are taken and if they're very low - meaning a sudden sharp fall, potentially related to DPD deficiency - then the patient will discontinue capecitabine immediately even before she has been on the drug for one week.
Efficacy of capecitabine in the metastatic setting
Currently, many of our patients receive anthracyclines and taxanes in the adjuvant setting, so an increasing number of patients will be treated with capecitabine even as first-line therapy. Most of the data we currently have is from patients who have already received anthracyclines and taxanes, and as we use capecitabine earlier we see more benefit.
Capecitabine is a potent agent. In the capecitabine/docetaxel versus single-agent docetaxel study led by Joyce O'Shaughnessy, the combination clearly proved to be quite potent as well. We all have questions about what would have happened if the single-agent docetaxel arm was followed with capecitabine. Would there have been equivalent survival and less toxicity? My guess is that overall survival would have ultimately been the same with a higher response rate and longer time to progression with the combination.
There aren't many studies that have truly tested a combination versus the same drugs in sequence. The best and largest study was the recently published ECOG-1193 trial evaluating doxorubicin and paclitaxel sequentially with crossover at progression versus the combination. The results were exactly as would be expected. The response rate was higher for the combination, but overall survival was identical at about 20 months in all three of the arms. Notably, patients treated with the combination had more toxicity.
Now, in patients with life-threatening disease in whom I am worried that if they don't respond to the first agent I won't have time to get a second one in, I start combination therapy up front. But many of my patients with metastatic disease don't have a lot of symptoms early on, so giving them the best quality of life is also really important.
Nonprotocol management of patients with HER2-positive metastatic disease
Generally, I start patients with HER2-positive disease on a taxane and trastuzumab. If I'm really trying to capitalize on synergistic combinations, it makes sense to add a platinum agent, knowing that synergy up front has improved survival.
Being in the Northwest, I have a lot of patients who are into holistic approaches and would prefer to delay introducing chemotherapy into their systems as long as possible. Thus, I frequently offer trastuzumab monotherapy as an option.
Response rates with first-line, single-agent trastuzumab are in the 35 percent range. We know from the pivotal trial that if you administer chemotherapy with trastuzumab, you do better than if you give chemotherapy followed by trastuzumab second-line. Even though 65 percent of the patients in the chemotherapy-alone arm ultimately received trastuzumab after they progressed, a five-month survival advantage was still demonstrated. Interestingly, however, we don't know whether giving trastuzumab alone up front and then adding chemotherapy at progression changes survival at all.
I discuss the data on combination and single-agent trastuzumab and tell patients that we actually don't know if it is better to give the combination up front or if there is any harm in giving trastuzumab alone and then adding the chemotherapy at progression. Generally, in a patient with life-threatening disease, I'm going to go for the best response and will recommend giving chemotherapy with trastuzumab. But for patients who have pretty low-volume or quiescent disease and are not symptomatic, or older patients in whom cardiac problems may arise, I think trastuzumab monotherapy is a reasonable option.
Select publications
|
| Dr Gralow is an Associate Professor of Medical Oncology at the University of Washington and Fred Hutchinson Cancer Research Center in Seattle, Washington. |
|
