| You are here: Home: BCU 2|2004: I Craig Henderson, MD, FACP, FRCP

Edited comments by
I Craig Henderson, MD, FACP, FRCP |
Role of the aromatase inhibitors in the adjuvant setting
The aromatase inhibitors are now clearly viewed as the most effective and important adjuvant endocrine therapy. In the last three or four years, we've seen an unexpected shift from tamoxifen, the "star" for 30 years, to the aromatase inhibitors. After the presentation of the initial ATAC trial results, ASCO did not recommend the aromatase inhibitors as adjuvant therapy because there were no survival data. Interestingly, the FDA approved anastrozole as adjuvant therapy. It's also clear to me that community-based doctors are using adjuvant anastrozole to a greater extent than most academic physicians.
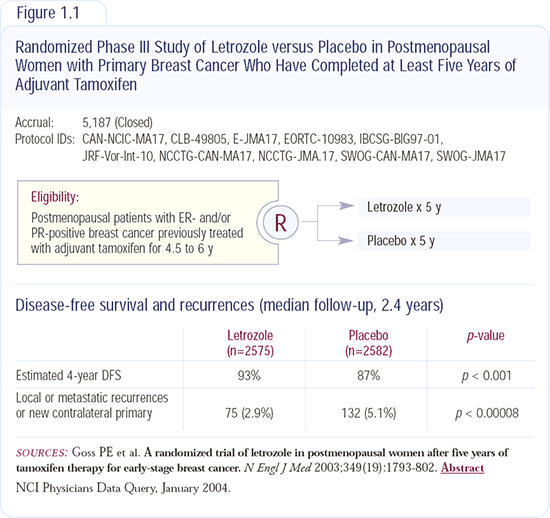
The dramatic results from the NCIC-CAN-MA17 trial (Figure 1.1) of letrozole after tamoxifen have thrown everyone into turmoil. The levels of significance are so great that neither physicians nor patients can ignore them. Again, we don't have survival data, and it will be difficult to evaluate survival at any point in the future. Additionally, we won't be able to replicate those results because it wouldn't be ethical to repeat that study. In fact, the NSABP trial evaluating exemestane in postmenopausal patients with receptor-positive breast cancer, which was identical in design, was closed to accrual immediately. I don't think it's possible to ignore the ATAC trial results anymore.
Disease-free survival and survival as endpoints in adjuvant trials
Disease-free survival and survival are important endpoints for patients. Patients asked to weigh these two endpoints invariably rate survival as the most important; however, when two treatments offer no difference in survival, patients face a difficult decision. I'm concerned, as are others, that neither the ATAC trial nor the MA17 trial will provide clear answers about survival.
Aromatase inhibitors following five years of adjuvant tamoxifen
It may be reasonable to offer an aromatase inhibitor to patients who completed a five-year course of adjuvant tamoxifen as long as five or 10 years previously. However, with every year that passes, the absolute risk of recurrence decreases; therefore, the risk-to-benefit ratio changes. Every year, the risks become more important relative to the benefit. As the risk of recurrence decreases, the toxicities of therapy become much more important.
Italian Tamoxifen Arimidex® (ITA) trial: Adjuvant anastrozole following two years of adjuvant tamoxifen
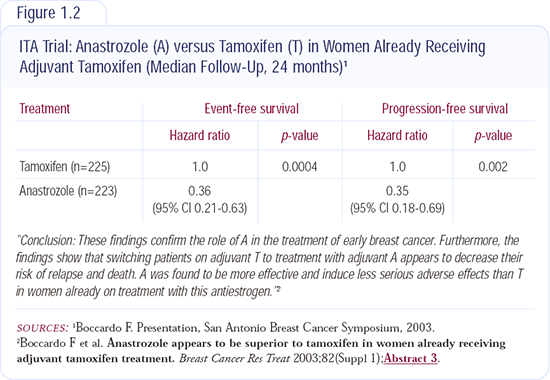
In the ITA trial (Figure 1.2), patients received a total of five years of therapy - either tamoxifen alone or tamoxifen for at least two years followed by anastrozole. Results from the ITA trial confirm the data from the MA17 trial in which patients received five years of adjuvant tamoxifen and then an aromatase inhibitor. It is unknown whether 10 years of an adjuvant aromatase inhibitor alone would be more effective than five years of adjuvant tamoxifen followed by five years of an adjuvant aromatase inhibitor. Although the ITA trial was a small study, I'm willing to accept it as being fundamentally correct because the results are consistent with those from the MA17 trial. In both trials, a clear advantage was demonstrated for the crossover to an aromatase inhibitor after tamoxifen.
Initiating adjuvant hormonal therapy
In a postmenopausal woman for whom I am initiating adjuvant hormonal therapy, I am now more likely to start with an aromatase inhibitor. I wouldn't rule out the possibility of tamoxifen, and I would discuss both options with the patient. If the patient asks what I recommend, I say an aromatase inhibitor. This is a recent change for me; the MA17 trial was the "final nail."
Continued adjuvant therapy following five years of adjuvant anastrozole
Kent Osborne proposed the possibility of studying the use of adjuvant tamoxifen in women who have already received five years of adjuvant anastrozole. Most physicians are concerned about this strategy because there are no data to support it. Another option would be 10 years of an adjuvant aromatase inhibitor. Most physicians seem to be more comfortable with that strategy.
Switching to adjuvant anastrozole while receiving adjuvant tamoxifen
In a postmenopausal woman who has received two to three years of adjuvant tamoxifen and is doing well, I wouldn't recommend changing to adjuvant anastrozole. I would tend to have the patient finish the five years of adjuvant tamoxifen and then change to an aromatase inhibitor. If the patient felt strongly about switching or was having some symptoms on tamoxifen, I'd be very comfortable switching therapy at two or three years.
BCIRG-001: Adjuvant TAC versus FAC
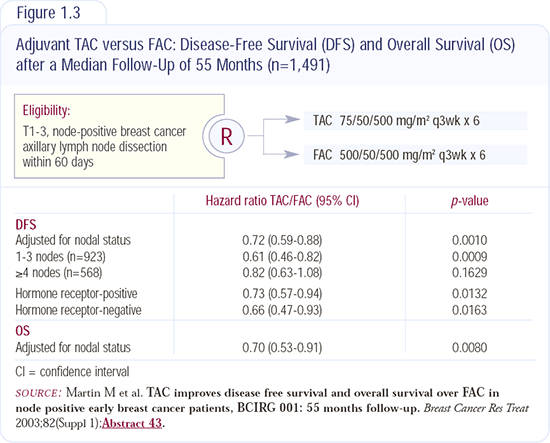
The TAC data were not surprising (Figure 1.3); I expected them to become positive for survival and disease-free survival. The analysis was very clear - no question -TAC is better than FAC. Now, the question is: Is the dose-dense regimen presented by Marc Citron last year, of AC every two weeks for four cycles with growth factors, followed by dose-dense paclitaxel for four cycles, better or worse than TAC?
The trial comparing TAC to FAC utilized an intravenous FAC regimen, but we've known for a long time that the SWOG FAC regimen is probably better. SWOG FAC uses daily oral cyclophosphamide, which prolongs its administration compared to the all-intravenous regimen. As established by randomized trials, classic CMF using oral cyclophosphamide is superior to an all-intravenous CMF regimen. Therefore, it's even more plausible that classic FAC would be better than the all-intravenous FAC regimen. Although TAC is better than intravenous FAC, it cannot be concluded that TAC is better than the SWOG FAC regimen.
Role of adjuvant docetaxel
Adjuvant AC followed by docetaxel is being used by many oncologists in practice, but we don't know how it compares to dose-dense AC followed by paclitaxel. Indirect evidence suggests that docetaxel is better than paclitaxel. A direct comparison between paclitaxel and docetaxel administered every three weeks in patients with metastatic breast cancer, presented at the 2003 San Antonio Breast Cancer Symposium, demonstrated a survival advantage for docetaxel (Figure 1.4).
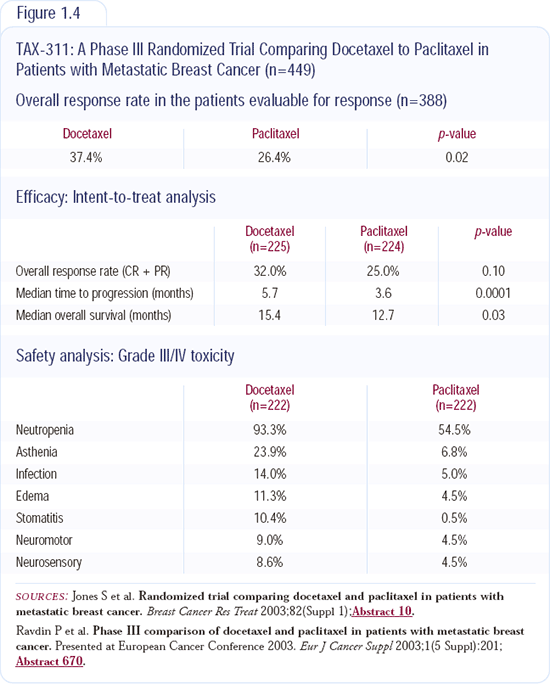
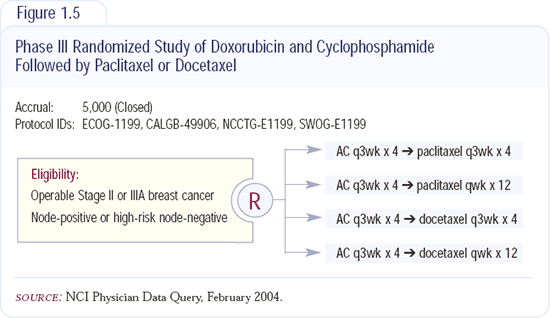
The data from the randomized Intergroup adjuvant trial will be reported in the next 18 to 24 months, and I will wait to draw a final conclusion at that time (Figure 1.5). In that trial, which is closed to accrual, patients were randomly assigned to either paclitaxel or docetaxel and to either an every three-week regimen or a weekly regimen. I believe paclitaxel may be better when administered weekly, and docetaxel may be better when administered every three weeks. It will be interesting to see how weekly paclitaxel will compare to every three-week docetaxel.
Selection of adjuvant chemotherapy
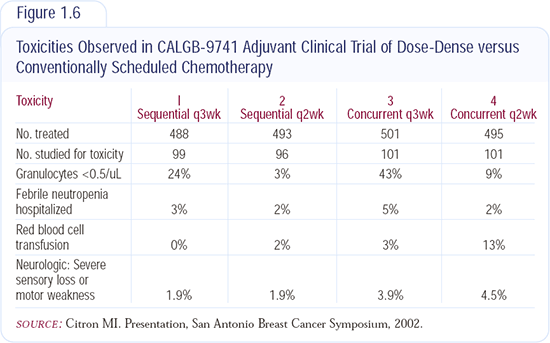
The most effective regimens are perceived to be TAC and dose-dense AC followed by paclitaxel. Without a comparative trial, it's difficult to say whether one is better than the other. A direct comparison is required to obtain a clear answer. I am most likely to use dose-dense AC followed by paclitaxel, but I helped to develop that regimen and we often use what we have the most experience with (Figure 1.6).
I believe Marc Citron and Cliff Hudis were surprised that dose-dense therapy wasn't more toxic; they feel that the dose-dense regimen is less toxic than the every three-week regimen, and their data support that.
Adjuvant chemotherapy in patients with node-negative disease
Unlike many of my colleagues, my recommendations for selection of a chemotherapy regimen in patients with node-negative disease are the same as for a patient at high risk. I believe that if you're going to use chemotherapy and expose the patient to the toxicities, you should do it right. The estimated three-year survival advantage at 10 years that physicians generally discuss with patients is based on the recent regimens. If you discuss those numbers with patients and then treat them with a less toxic regimen, like CMF or four cycles of AC, I consider that "bait and switch." Those regimens do not provide the benefit that was quoted.
Influence of estrogen-receptor status on the use of adjuvant chemotherapy
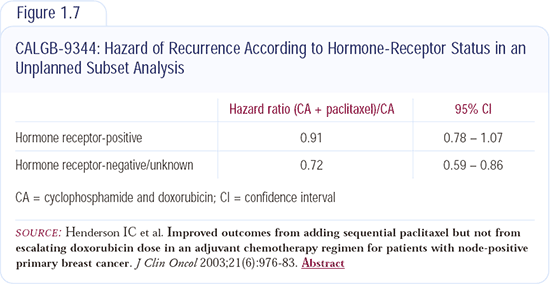
I probably use less chemotherapy in postmenopausal women than many of my colleagues, although I've recently increased my usage. I wouldn't have treated any 60-year-old women with adjuvant chemotherapy five years ago, but I've made a real change since the results from CALGB-9344 were published (Figure 1.7). The effects of chemotherapy are usually reported in all patients - those with ER-positive disease and those with ER-negative disease - but evidence suggests that chemotherapy is less effective in patients with ER-positive disease and more effective in patients with ER-negative disease.
I'm now convinced that the effect of chemotherapy in a 60-year-old woman with ER-negative disease is the same as in a 45-year-old premenopausal woman with ER-negative disease. I would treat that 60-year-old woman with chemotherapy, and I would give her the best chemotherapy available.
On the other hand, in an otherwise healthy 60-year-old woman with a 2.5-cm, moderately well-differentiated tumor that has 80 percent estrogen receptor staining and 40 percent progesterone receptor staining, I would very likely use adjuvant endocrine therapy alone. I would discuss and offer chemotherapy,
particularly if she had node-positive disease. If the patient asked, "What do you recommend?" I'd say, "Most of your benefit is going to come from the endocrine therapy, and you're going to possibly obtain a little benefit from chemotherapy in the range of one-half to one-and-a-half percent. If you want to be treated, that is fine." My approach would be different in a woman with ER-negative disease because I would give a much higher estimate of the benefit from chemotherapy.
Select publications
|
| Dr Henderson is an Adjunct Professor of Medicine at the University of California in San Francisco, California. |
|
