| You are here: Home: BCU 2|2004: Anthony Howell, MD, MSc, FRCP

Edited comments by
Anthony Howell, MD, MSc, FRCP |
ATAC trial findings and hormone receptor phenotype
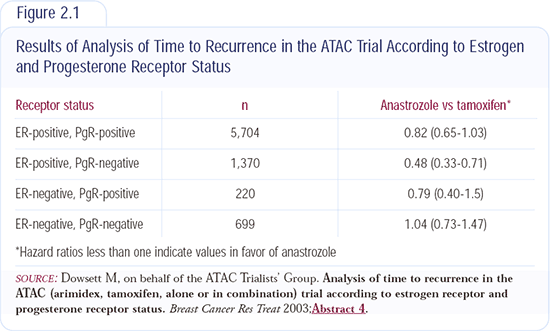
The analysis of recurrence according to estrogen and progesterone receptor status was the first translational research component of the ATAC trial to be reported. The data indicate patients with ER-positive and PR-negative tumors - approximately 20 percent of postmenopausal ER-positive patients with breast cancer - have a 50 percent reduction in the hazard for recurrence with anastrozole compared to tamoxifen, whereas those with ER/PR-positive tumors have about a 20 percent reduction in the hazard ratio (Figure 2.1).
We need to be cautious because these are early data and it's the first time this pattern has been reported. Biologically, it makes sense because patients with ER-positive, PR-negative disease tend to be HER2-positive in other trials with which we've been involved. Additionally, in the letrozole preoperative trial and the IMPACT neoadjuvant anastrozole trial, patients with ER-positive, HER2-positive disease responded better to aromatase inhibitors than to tamoxifen.
HER2 status and response to endocrine therapy
We haven't yet evaluated HER2 status in the ATAC trial, but in the NATO trial 30 percent of patients with ER/PR-negative disease were HER2-positive, whereas only 10 percent of those with ER/PR-positive disease were HER2-positive. We believe there's an association between HER2 and ER. Activation of growth factor receptors may turn off progesterone receptor synthesis.
The hypothesis that PR is a downstream function of ER and an indication of how ER is functioning is also reasonable. In advanced disease, patients with ER/PR-positive disease are more likely to respond to endocrine therapy than those with ER-positive, PR-negative disease.
However, these observations have all been with tamoxifen, and data first presented at the 2003 San Antonio Breast Cancer Symposium suggest the aromatase inhibitors may be more effective than tamoxifen in patients with the ER-positive, PR-negative phenotype.
This is a potentially important finding, but it appears that the aromatase inhibitors are also more effective for patients with ER/PR-positive disease.
Current limitations in measuring and definining ER positivity
The patient subset with ER-negative, PR-positive tumors has been recognized for many years, and there are different viewpoints about this subgroup. It's a very small group of patients - only about 250 out of the 6,000 patients we looked at in the ATAC trial had that phenotype. In older trials of advanced disease, these patients responded to tamoxifen, so it's not a reason for failing to offer endocrine therapy. The estrogen receptor is almost certain to be present at very low levels, but we're not measuring it.
Assessment of ER status remains problematic. In the past, assays were standardized by biochemical methods that were widely utilized. The immunohis-tochemical method can be performed in any pathology laboratory, but quality control is poor in some laboratories.
The real problem with false-negative results occurs for tumors with low levels of ER - between one and 20 percent of positively staining cells - which comprises 10 percent of patients. The concern is that these patients will be labeled ER-negative and will not receive the benefit of endocrine therapy.
Another concern is that we don't know how patients with low levels of ER respond to therapy, although the IMPACT trial had a lower response rate for patients in the lowest quartile of positivity. Having said that, these patients do respond to endocrine therapy. I believe we should be administering endocrine therapy to all patients who demonstrate any ER positivity.
Many laboratories are beginning to utilize the Allred scoring system, which is good because the Baylor group has the most data on the immunohistochemical measurement of ER.
ATAC trial subprotocol analyses: Quality of life and side-effect profiles
The quality-of-life subprotocol resulted in no difference between tamoxifen and anastrozole; however, anastrozole was more effective in preventing relapse.
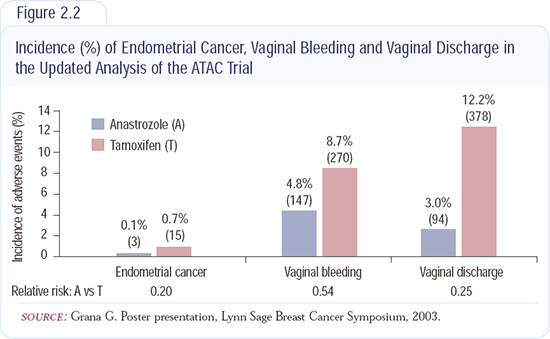
Other important differences favoring anastrozole over tamoxifen were fewer strokes, deep vein thromboses, heart events and endometrial cancers (Figure 2.2). These extremely important advantages of anastrozole outweigh the relatively minor side effects - aching in the joints and dryness in the vagina.
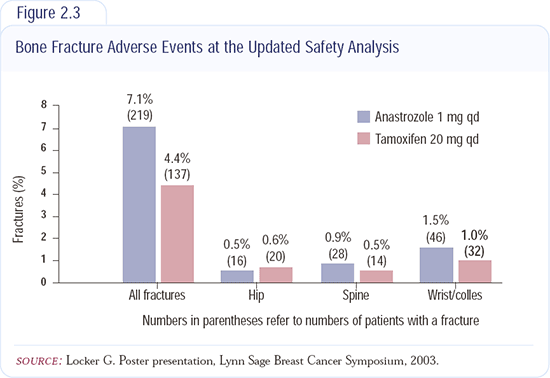
The major side effect associated with anastrozole is the decrease in bone mineral density. The bone subprotocol - out to two years - shows a greater reduction in bone density with anastrozole than with tamoxifen (Figure 2.3). There's about a four percent bone loss with anastrozole and no bone loss with tamoxifen.
Use of bisphosphonates with adjuvant anastrozole
Increasingly, we're seeing patients who have ER-positive tumors and good prognoses, so we have to think of their global quality of life for the remainder of their lives. One could argue that assessing bone density in women over 60 is a public health measure, irrespective of whether they have breast cancer or not.
In a nonprotocol setting, I think we should measure bone density and prescribe bisphosphonates if necessary. The Austrian data, which are highly important, are holding up and demonstrate that bone mineral density loss associated with anastrozole can be prevented with the bisphosphonate zoledronate.
We don't really know which bisphosphonate to use, although it's likely any of the good bisphosphonates will be efficacious. The IBIS-II prevention trial comparing anastrozole to placebo has a bone subprotocol in which we will measure the baseline bone mineral density in 900 women, with repeat assessments at one, three, five and seven years. If their bone density is normal at entry, we won't intervene. If they have osteoporosis, we'll treat them with weekly risedronate.
The patients with osteopenia are interesting because they are the ones who are likely to be tipped into osteoporosis during the five years of treatment with an aromatase inhibitor. In the United Kingdom, patients with osteopenia would be treated by their family doctors and would not receive a bisphosphonate until they developed osteoporosis. In the IBIS-II study, those patients will be randomly assigned to weekly risedronate or placebo.
ASCO Technology Assessment on the Use of Adjuvant Aromatase Inhibitors
It's interesting that relatively small improvements from chemotherapy have been accepted, whereas the ASCO Technology Assessment was equivocal with regard to the adjuvant use of anastrozole. Unlike other clinical trial results, they suddenly wanted to see a survival advantage. One could make the argument for the use of aromatase inhibitors due to the delay in relapse and the side-effect profile, particularly with regard to the endometrium and deep vein thrombosis. Anastrozole offers other advantages, and I believe that a survival advantage will become evident in time. The first analysis of survival data will likely occur in the summer of 2004.
More data are being reported on aromatase inhibitors. The MA17 trial demonstrated the value of aromatase inhibitors after five years of tamoxifen, and the Boccardo trial indicated that the switch from tamoxifen to anastrozole at two or three years from the start of treatment results in a disease-free survival advantage and nearly a survival advantage, with a p-value of 0.06. Increasingly, more data are emerging to support the superiority of aromatase inhibitors over tamoxifen.
Selection of an aromatase inhibitor in the adjuvant setting
A good scientist and clinician will treat patients according to the available data. In the adjuvant setting, the ATAC data support using anastrozole up front, the Boccardo data support switching to anastrozole after two to three years of tamoxifen, and MA17 supports the use of letrozole after five years of tamoxifen.
So at this point, if you are starting adjuvant therapy, you should use anastrozole because we have data on that. If you are going to switch at two to three years, you switch to anastrozole because we have data on that. But if you're going to give treatment after five years, you use letrozole because we have data on that.
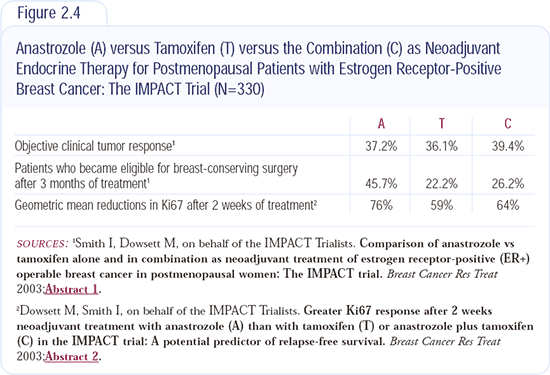
IMPACT neoadjuvant trial: Anastrozole versus tamoxifen versus the combination
The IMPACT trial can be thought of as preoperative ATAC, with treatment given for three months. Response rates were similar in all three arms - approximately 30 percent by calipers - but breast conservation rates were significantly higher with anastrozole (Figure 2.4).
In the biological study reported, anastrozole resulted in approximately a 20 percent reduction in the proliferation index Ki67 compared to either tamoxifen or the combination. These results were similar to the ATAC trial results. Additionally, the response rate was higher in patients with HER2-positive disease, which mirrors Matt Ellis' data with letrozole.
Role of fulvestrant in the sequence of hormonal therapies
In trials 20 and 21, anastrozole and fulvestrant were equivalent as second-line therapy after tamoxifen failure, but fulvestrant had a significantly longer duration of response in the North American study. In the first-line study, tamoxifen was slightly superior to fulvestrant, which was a very surprising result. In the ER/PR-positive group, fulvestrant was slightly (but not significantly) better than tamoxifen. In other words, it's a drug that is equivalent to anastrozole as second-line therapy and nearly equivalent to tamoxifen as first-line therapy.
We have to ask, "Why wasn't fulvestrant better than tamoxifen?" That's what we expected. The answer may be in the dosing of fulvestrant, because it takes about six months to achieve steady state levels.
Clinical trials (Figure 2.5) will evaluate loading-dose schedules of fulvestrant. Our modeling analyses indicate these approaches will increase the dose of the drug sooner, and then we will be able to investigate whether that is the reason fulvestrant was not better than tamoxifen in the first-line trials.
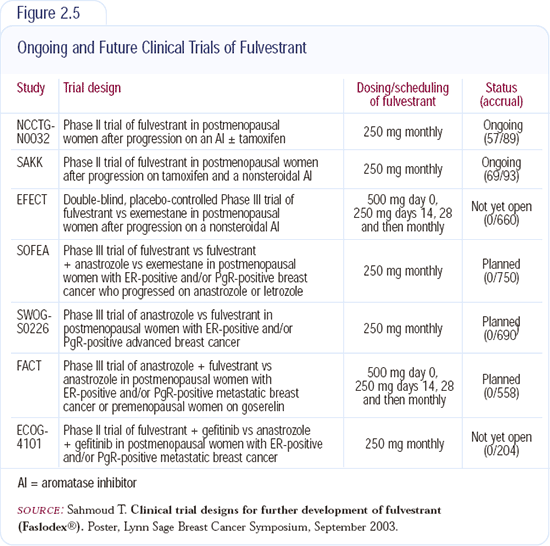
It remains unclear where fulvestrant should be utilized in the sequence of hormonal therapies for metastatic disease. Several new North American trials and the SOFEA trial should help to clarify its role in our armamentarium of hormonal therapies. The SOFEA trial is a three-arm comparison between exemestane, fulvestrant and fulvestrant plus anastrozole after progression on a nonsteroidal aromatase inhibitor. It's possible that by discontinuing the aromatase inhibitor, sufficient estrogen will be produced to circumvent the effects of fulvestrant. The SOFEA trial will provide an indication of whether fulvestrant is better than exemestane as second-line therapy and also whether it's necessary to suppress the levels of estrogen.
Research strategies for the chemoprevention of breast cancer
The ATAC, Boccardo ITA and MA17 trials demonstrated dramatic reductions in contralateral breast cancer in patients receiving an aromatase inhibitor compared to tamoxifen. We estimate tamoxifen provides about a 50 percent reduction in contra-lateral breast cancer, whereas anastrozole may result in a 70 to 80 percent reduction, so it's logical to consider using an aromatase inhibitor for prevention.
The IBIS-II trial will compare anastrozole with placebo in 6,000 women at high risk for the development of breast cancer. We did not include tamoxifen as the comparator because we were concerned about tamoxifen's side-effect profile.
Select publications
|
| Dr Howell is a Professor of Medical Oncology at the University of Manchester in Manchester, England. |
|
