| You are here: Home: BCU 3|2004: Francesco Boccardo, MD

 |
Edited comments by
Francesco Boccardo, MD |
 |
Rationale for the use of aromatase inhibitors following adjuvant tamoxifen
The newer aromatase inhibitors are as effective as, if not better than, tamoxifen as first-line therapy for advanced disease. They do not affect the uterus or increase the risk of thrombo-embolic disease. On the other hand, aromatase inhibitors can lead to osteoporosis. As reported in the ATAC trial, the aromatase inhibitors are associated with an increased incidence of fractures.
Approximately 10 to 12 years ago we began exploring the role of adjuvant aromatase inhibitors following a course of adjuvant tamoxifen. Although adjuvant tamoxifen is very effective, it is not devoid of serious side effects. Attention to the possible mechanisms of tamoxifen resistance was also growing. One particular mechanism of resistance, an increase in aromatase activity in the breast tumors of women exposed to tamoxifen, provided strong biological support for this sequencing approach.
We believed a sequential approach could have potential advantages over a five-year course of adjuvant tamoxifen or even an adjuvant aromatase inhibitor. A sequential approach would allow women to receive a class of compounds that might help circumvent tamoxifen resistance while limiting the exposure to aromatase inhibitors and costs of treatment.
Trial evaluating three years of adjuvant tamoxifen followed by two years of adjuvant aminoglutethimide
In 1992, aminoglutethimide was the only aromatase inhibitor available, and we began a sequencing trial with it. Based on a prior adjuvant trial by Coombes, in which approximately 25 percent of women treated with aminoglutethimide plus hydrocortisone discontinued treatment due to side effects, we selected a low dose of aminoglutethimide.
In our trial, 380 postmenopausal women who had completed three years of adjuvant tamoxifen were randomly assigned to two more years of tamoxifen or 250 mg of aminoglutethimide. Most of the women had ER-positive, node-positive disease. Although no difference was found in the overall recurrence rate, a difference in the sites of recurrence was observed — more visceral recurrences were seen in the patients who continued on tamoxifen. Additionally, patients who switched to aminoglutethimide had a significantly longer survival. This difference was probably related to an increase in both breast cancer-unrelated and breast cancer-related deaths in the patients continuing on tamoxifen.
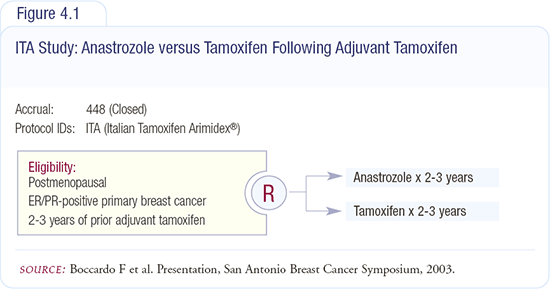
Italian Tamoxifen Arimidex® (ITA) trial
When anastrozole became available in 1998, we designed a companion trial to our aminoglutethimide study that was similar in design to allow for a pooled analysis of the data from the two trials. The new trial, known as the Italian Tamoxifen Arimidex® (ITA) trial (Figure 4.1), substituted anastrozole for aminoglutethimide and restricted enrollment to postmenopausal women with ER-positive, node-positive breast cancer.
Following treatment with two to three years of adjuvant tamoxifen, 448 women were randomly assigned to continue tamoxifen or switch to anastrozole for a total of five years of adjuvant therapy. The treatment groups were balanced with respect to median age, tumor size and grade, number of involved nodes, type of primary treatment, and prior radiation therapy or chemotherapy. The median age for both groups was 63 years. The median duration of tamoxifen therapy prior to randomization was 28 months in each group.
After a median follow-up of three years, 17 recurrences occurred in the women who switched to anastrozole and 45 recurrences occurred in the women who continued on tamoxifen. The women who continued on tamoxifen had more second primary tumors (including five endometrial cancers), more distant metastases and more locoregional recurrences (including ipsilateral breast, loco-regional node recurrences, or both). According to the Kaplan-Meier curves, the women who switched to anastrozole had a significantly longer event-free, progression-free and local relapse-free survival. They also had a longer, although not significant (p = 0.06), distant metastases-free survival. Overall survival (p = 0.1) was also longer for the women who switched to anastrozole, but there were few deaths because the data are immature.
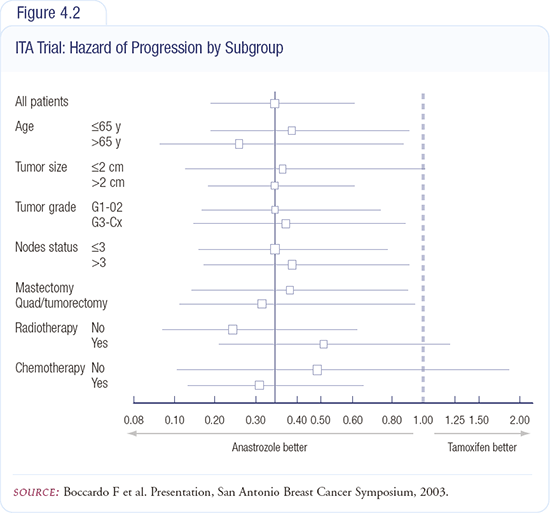
The treatment discontinuation rates for both groups were similar (8.4 percent for tamoxifen and eight percent for anastrozole). Women who continued on tamoxifen exhibited significantly more gynecologic changes, many of which were serious and required hospitalization. More severe treatment-related adverse events were reported in the women who continued on tamoxifen (Figure 4.2).
Implications of the recent adjuvant aromatase inhibitor trials
Given its relatively small size and immature data, we should avoid overinterpreting the results from the ITA trial. However, these data together with previous data support an advantage for switching adjuvant therapy. The data on switching adjuvant therapy are consistent with the data from the ATAC and MA17 trials. In the MA17 trial comparing letrozole to placebo in women who had received five years of adjuvant tamoxifen, placebo may have potentially represented active therapy since it is postulated that tamoxifen may become a stimulatory growth factor. Hence, it has been hypothesized that some of the women who discontinued adjuvant tamoxifen after five years might have had a withdrawal response.
We don’t truly know which of our patients will benefit from an adjuvant aromatase inhibitor. Only a small proportion of women treated with anastrozole in our trial or the ATAC trial or treated with supplementary letrozole in MA17 actually benefited. I would probably be a bit conservative in applying these trial data, since mortality is the primary endpoint for adjuvant therapy. We can select women who are not candidates for tamoxifen who would benefit from an aromatase inhibitor. For women with progressive thickening of the endometrium or tamoxifen intolerance it might be prudent to consider switching to an aroma-tase inhibitor.
Nonprotocol role of adjuvant aromatase inhibitors following a two- to five-year course of adjuvant tamoxifen
In some specific subsets of women, it is appropriate to switch from tamoxifen to an aromatase inhibitor after two or five years of tamoxifen. There is no reason to continue tamoxifen in women who may be at risk for problems with tamoxifen, because we now have alternatives. The evidence is not yet compelling to state that an aromatase inhibitor should be substituted for tamoxifen, and I am not in a position to make a recommendation that will affect so many thousands of women.
At the moment, if patients are tolerating tamoxifen well, have undergone hysterectomy and are not at risk for thromboembolic events, I’m not likely to recommend switching to an aromatase inhibitor.
In the future, I expect most, if not all, women will receive adjuvant therapy with aromatase inhibitors, and many of them will be treated empirically for the risk of osteoporosis.
Select publications
|
| Dr Boccardo is a Full Professor of Medical Oncology at the University and National Cancer Research Institute in Genoa, Italy |
|
