| You are here: Home: BCU 3|2004: John F R Robertson, MD, FRCS

 |
Edited comments by
John F R Robertson, MD, FRCS |
 |
Potential strategies to improve the efficacy of fulvestrant
Fulvestrant 250 mg is an effective dose, as demonstrated by the clinical trials. It is as effective as anastrozole as second-line therapy and equivalent to tamoxifen as first-line therapy in postmenopausal women. In premenopausal women, data suggest that 250 mg of fulvestrant is not effective at downregulating the estrogen receptor. This raises questions about whether a 250 mg dose of fulvestrant leads to complete down-regulation of the estrogen receptor in postmenopausal women. Could a higher dose of fulvestrant achieve more?
Two strategies exist to increase the dose of fulvestrant. The first is a loading dose sequence. The second is the administration of a higher dose of fulvestrant. For example, instead of administering one five-milliliter injection every month in one buttock, using one five-milliliter injection in each buttock, for a total of 500 mg. Future studies are needed to determine the dose-response curve for fulvestrant.
Trials combining fulvestrant with an aromatase inhibitor
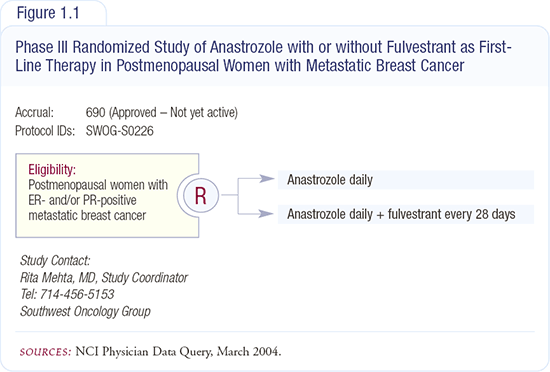
Either increasing the fulvestrant dose or decreasing estradiol levels can evaluate the dose-response curve for fulvestrant. A number of studies are beginning to look at decreasing estradiol levels with aromatase inhibitors. In my own unit in Nottingham, we are randomly assigning patients preoperatively to three weeks of fulvestrant, anastrozole or the combination. SWOG-S0226 will compare anastrozole to anastrozole plus fulvestrant as first-line therapy in postmeno-pausal women (Figure 1.1).
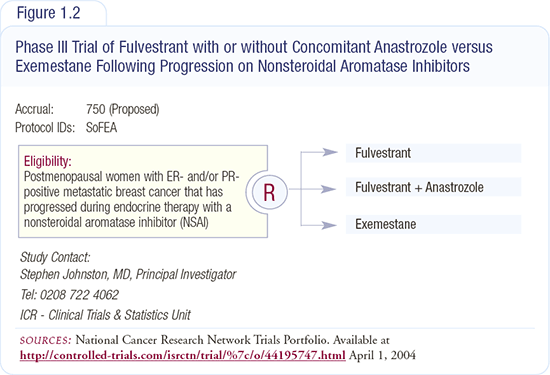
In the UK, the SoFEA study (Figure 1.2) will enroll patients who have had disease progression while on an aromatase inhibitor. Those patients will be randomly assigned to fulvestrant, exemestane or fulvestrant plus anastrozole. The rationale behind that trial is the data suggesting that estrogen-deprived MCF-7 cells become supersensitive to lower doses of estradiol and, hence, are stimulated again. The third arm of that trial will keep the estradiol levels low and then come in with fulvestrant to determine if that strategy is different from fulvestrant alone without estradiol suppression.
Duration of response to hormonal therapy
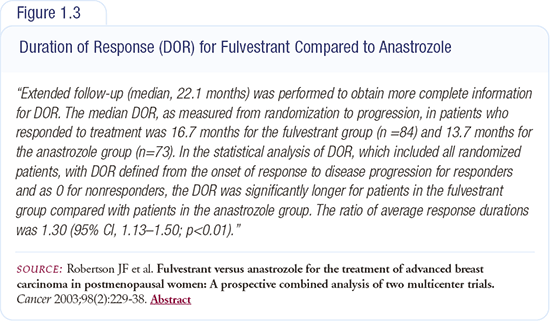
Retrospective data suggest that fulvestrant may have a longer duration of response than anastrozole (Figure 1.3). It’s an interesting finding that would support some of the preclinical models. However, as academic clinicians, we need to be rigorous in our review of the data.
Our group has some patients who have had long durations of response to fulvestrant. One woman was on fulvestrant for more than seven years, another for more than five years and another for four and a half years. We’ve also reported good responders who were treated with other hormonal agents. The only way to test whether patients treated with fulvestrant have longer durations of response is by conducting a randomized trial.
Tolerability of fulvestrant injections
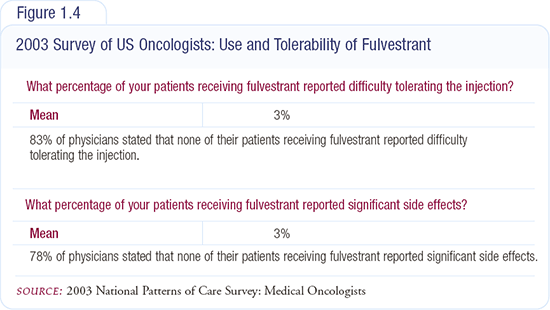
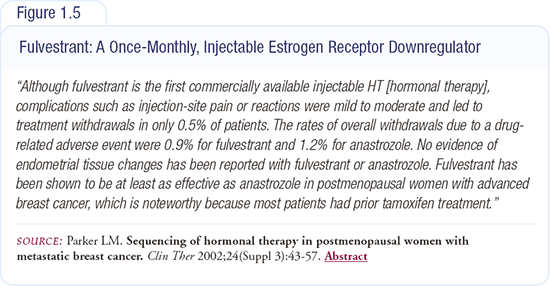
I don’t believe that fulvestrant injections are a major problem. We have been administering fulvestrant for nearly 10 years and have not had any serious problems with the injection — no patients with sterile abscesses or complaining of buttock pain (Figures 1.4 and 1.5). The clinical trials have demonstrated similar favorable results. In women being treated with a bisphosphonate, fulvestrant provides the opportunity to have their treatment completed during a single visit each month.
Alterations in breast cancer cell phenotype after tamoxifen therapy
We’ve been collecting tumor samples from patients before tamoxifen therapy, after six weeks and six months of tamoxifen therapy, and at progression. Initially, downregulation of the estrogen and progesterone receptors occurs; then, when the cancers become resistant, quite marked levels of estrogen and progesterone receptors are present. During response to tamoxifen, a downregulation in proliferation occurs, and at progression, an upregulation in proliferation is evident.
We have not seen a huge upregulation of growth factors, like HER2 and EGFR, using older assays. We’re about to launch a new study using microarrays on the samples we’ve collected. We’re also going to use the new assays for EGFR and HER2. It will be interesting to see if those pathways are also being upregulated. Studies with tamoxifen-resistant cell cultures have demonstrated an upregulation of growth factors and sensitivity to gefitinib.
Phase II trial of gefitinib in patients with tamoxifen-resistant or ER-negative breast cancer
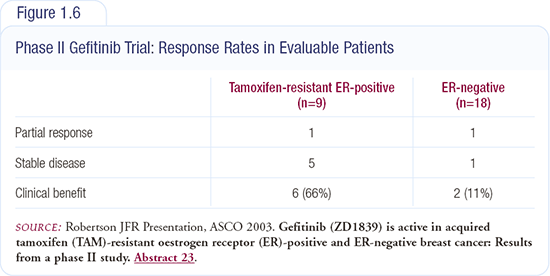
At ASCO 2003, we presented results from a Phase II trial of gefitinib in patients with metastatic breast cancer who were not heavily pretreated. The two arms of the trial included: (1) patients with tamoxifen-resistant breast cancer who had only been treated with tamoxifen and (2) patients with ER-negative breast cancer who had only received one prior chemotherapy regimen. We reported an 11 percent clinical benefit rate in the patients with ER-negative disease and a 66 percent clinical benefit rate in patients with tamoxifen-resistant breast cancer (Figure 1.6).
Gefitinib has not yet been evaluated in patients with previously untreated breast cancer. However, hormone-sensitive MCF-7 cells that have not been exposed to tamoxifen do not respond to gefitinib. Cell culture data suggest that the growth factor pathways are activated when cells become tamoxifen resistant.
Response to gefitinib in a patient with tamoxifen-resistant breast cancer
One of the patients I enrolled in the Phase II gefitinib trial had liver metastases, which have been in complete remission for almost 21 months. This postmenopausal woman was treated with a mastectomy a number of years ago. Then, she received tamoxifen for the treatment of liver and bone metastases. She responded well to tamoxifen and then progressed. At that point, she was in her late seventies and we felt it was reasonable to try gefitinib alone rather than chemotherapy. Within three months, she had a complete response in the liver metastases and a partial response in the bone metastases, and she’s currently still being treated. She experienced a few side effects — the classic skin rash, lethargy and alopecia. Her skin rash resolved when we reduced the gefitinib dose from 500 mg/day to 250 mg/day and her hair started to grow back while continuing on gefitinib.
Combining hormonal and biologic agents
A study that is about to start will compare tamoxifen to tamoxifen plus gefitinib. Preclinical data have shown that in the same way gefitinib can treat tamoxifen resistance, when it is administered initially, gefitinib seems to prevent resistance. This clinical trial will evaluate whether gefitinib can prevent or delay acquired clinical resistance in patients with metastatic breast cancer. I believe a study should also evaluate the efficacy of gefitinib after fulvestrant. Preclinical data suggest that gefitinib may reverse or prevent tamoxifen resistance; if it also reverses or prevents fulvestrant resistance, then gefitinib may affect a whole group of patients.
If we can establish that gefitinib is useful for either the treatment or prevention of endocrine resistance, it will be a major addition to our armamentarium. The same may potentially be true for trastuzumab. I believe that a trial should be conducted with tamoxifen and trastuzumab or with tamoxifen, trastuzumab and gefitinib. Fulvestrant and trastuzumab is another possible combination.
Utility of tumor markers in patients with breast cancer
The established antigen-based tumor markers are cancer antigen CA 15-3 and carcinoembryonic antigen (CEA). For a number of years, we’ve known that cancer can be detected earlier in patients followed with these markers. At least two pilot studies have now shown that not only can cancer be detected, but if early intervention is utilized, outcomes can be affected.
One study from Germany demonstrated that patients with elevated tumor markers and no evidence of metastases on scans, who were randomly assigned to a change in treatment, experienced a delay in the onset of symptomatic metastases. No impact on survival was found, but a marked delay in onset of symptomatic metastatic disease was evident. A study from Italy with a similar design showed not only a delay in the onset of symptomatic metastases, but also an improvement in survival from the time of the primary surgery.
We’re hoping to initiate a study that will randomly assign patients to standard follow-up, or routine tumor marker follow-up in addition to standard follow-up as a basis for early intervention. I have called it the “Second Adjuvant Therapy Study,” and it will be based on two other studies. The first is a substudy from the ATAC trial. We’ve been collecting follow-up blood samples from a subpopulation of those patients to determine the patterns of change in tumor markers that indicate whether a patient will develop metastatic disease. Hence, we hope to learn when a patient’s treatment should be altered.
The second is a study we’ve been conducting that is similar to the Italian trial —an early intervention study. We are evaluating the problems that can occur with such a study, because patients are certainly more anxious when they know their tumor markers are elevated without any obvious signs of metastases. Will that anxiety be offset by increased disease control?
Select publications
|
| Dr Robertson is a Professor of Surgery at the University of Nottingham in Nottingham, England. |
|
