| You are here: Home: BCU 3|2004: Gary H Lyman, MD, MPH, FRCP

 |
Edited comments by
Gary H Lyman, MD, MPH, FRCP |
 |
Survey of dose and schedule of adjuvant chemotherapy in practice
We contracted with over 1,200 non-academic practices of all sizes (but not academic centers) geographically distributed across the country. We asked them to gather information on their last series of patients receiving adjuvant chemotherapy for breast cancer, starting currently and going backward. These were patients who were treated with a mixture of chemotherapy regimens from the mid-1990s until early 2000. We are just beginning to evaluate patients treated more recently. Our report published in the Journal of Clinical Oncology focused on approximately six years of data from approximately 20,000 women. The primary area of interest was dose intensity (Figure 2.1).
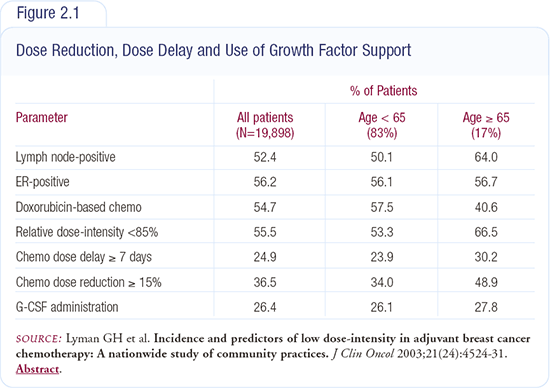
It was a very eye-opening experience. We found that the majority of women underwent some degree of reduced dose intensity from the published reference standards. In fact, 56 percent of women, across all regimens, are receiving less than 85 percent of targeted dose intensity.
In those patients experiencing dose reductions approximately 40 percent was planned dose reduction, which I believe reflects an intention to “go light” on the first cycle and then raise the doses for subsequent cycles if the patient tolerates therapy well. That seldom occurs, even in patients who don’t develop neutro-penic complications. It’s extremely rare for those cycle-specific dose intensities to be raised during subsequent cycles. Once started low, doses continue to remain low. In the unplanned reductions, we believe 60 to 65 percent are due to physician or patient responses to hematologic toxicities and 40 percent are due to non-hematologic complications.
Utilization of growth factor support
One variable that has changed over time is the use of growth factors. While I can’t overemphasize the limitations of retrospective chart reviews, growth factors are not commonly used early in adjuvant therapy of breast cancer, like they might be used in patients with lymphomas or those receiving more intensive regimens.
Approximately, one-fourth of patients in our study received a hematopoetic growth factor during the course of treatment, but 85 percent received it secondarily after toxicity occurred. Only two to three percent of patients received primary prophylaxis and those were probably elderly patients or patients with comorbidities.
Calculation of dose based on body surface area
Every oncologist has a threshold at which they become anxious and begin to adjust weight to ideal, or to some compromise between ideal and actual body weight. In our study, that threshold was extremely variable and particularly dramatic above 2.0 m2 body surface area (BSA). Many practices have patients with BSAs exceeding 2.75 m2, or even 3.0 m2, and calculating dose based on actual weight can arouse anxiety. However, for patients in whom dose was based on actual body weight, there was no greater hematologic toxicity or later dose reduction or treatment delay, at least not in patients with BSAs between 2.0 m2 and 2.3 m2.
My oncology group is excited about these findings and about trying to reevaluate the early data that served as the basis for our approach of dosing based on BSA. In reviewing those early studies, one realizes that a handful of patients were studied, with techniques that we could probably improve upon today. Were going back and trying to redo many of the early pharmacokinetic studies to determine if basing dose on BSA — of all the possible options that are out there — still seems to be the most rational approach. It seems to be how most physicians are currently calculating dose.
Impact of dose and schedule on long-term outcome
The impact of dose intensity on long-term outcome is our primary interest. We debated before we conducted this retrospective survey because we didn’t want physicians to dismiss the results as being from “selected” patients.
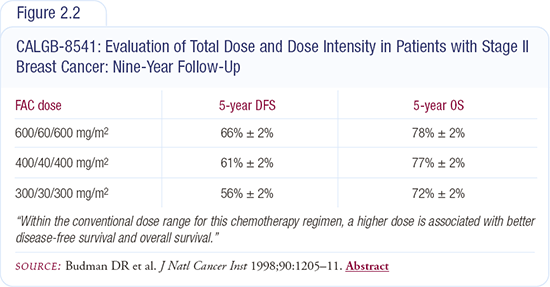
In breast cancer, we have data from the Budman-Wood study, which randomly assigned patients to three different relative dose intensities of CAF. This study was published initially in the New England Journal of Medicine in 1994 and then in the Journal of the National Cancer Institute in 1998 with nine years of follow-up (Figure 2.2).
A 50 percent reduction in relative dose intensity demonstrated a significant reduction in disease-free and overall survival at five years. The one-third reduction in dose intensity showed a significant decrease in disease-free survival, but overall survival was not yet significantly different.
Why might that be? Can we actually measure the impact of dose intensity and the impact on outcome in patients with a 10 percent, 15 percent, or even 25 percent reduction in relative dose intensity? This is where it becomes very difficult, because it’s largely a power issue. Those studies have not been done prospectively.
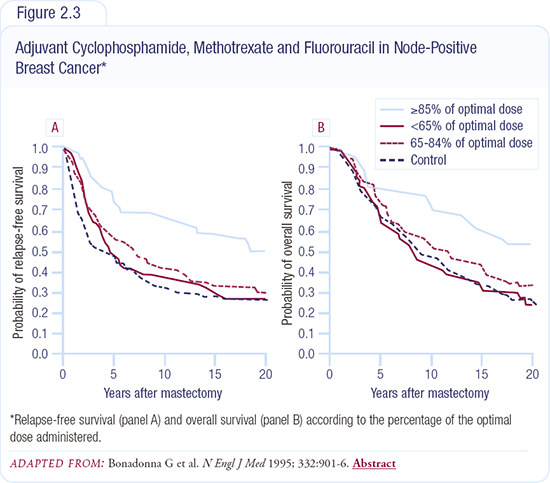
In 1995, Bonadonna retrospectively evaluated his CMF data and found enormous differences between women who received more than 85 percent of CMF dose intensity on a 28-day schedule versus those who received less than 85 percent (Figure 2.3). Patients who received less than 65 percent of standard dose had a disease-free and overall survival no different than that of the control group. The problem with Bonadonna’s study is that there are many other potential causes for those reduced dose intensities that might also be related to outcomes.
Retrospective data from the Toronto group and others almost always demonstrated that reducing dose intensity was associated with poorer outcomes, but we really need prospective randomized trials to resolve this issue. Despite the CALGB trial and a smaller French adjuvant trial — which evaluated FEC 100 versus FEC 50 and showed a significant decrease in disease-free and overall survival with the lower-dose epirubicin — the power calculations would indicate that you literally need thousands of patients in each arm of a trial to measure these kinds of small decrements.
Dose intensity delivered with dose-dense adjuvant chemotherapy
The timing of our survey results fall on the heels of reporting the results of CALGB trial 9741 and other data suggesting dose-dense regimens may provide a therapeutic advantage. We do not yet have data on patients who received dose-dense adjuvant chemotherapy in our survey, but the registry is tabulating that data. In my own experience — both in the trial setting and now in the post-trial setting — these patients seem to do extremely well and require very little compromise in their treatment dose intensity. Of course, they’re all receiving growth factor support, so there’s an economic issue that hasn’t been fully addressed. If the early differences in the arms from CALGB-9741 hold up over time, I think cost will become a secondary issue because dose-dense chemotherapy will result in a significant improvement in long-term outcome.
Algorithm for managing neutropenia during adjuvant therapy
The adjuvant chemotherapy regimens for early-stage breast cancer have not demonstrated an extremely high rate of febrile neutropenia. With AC every 21 days, the rates of febrile neutropenia are quite low but somewhat higher with the addition of a taxane. Severe neutropenia — less than 500 neutrophils at the nadir —is probably more common, although I dare say that many of my colleagues aren’t even looking at it today because the occurrence of febrile neutropenia is so low.
In 1998, Jeff Silber’s group published back-to-back papers in the Journal of Clinical Oncology, in which they developed a model based on retrospective analysis of 100 women receiving adjuvant breast cancer chemotherapy. They identified three factors in multivariate analyses that were significant predictors of future dose reductions, treatment delays or neutropenic events that would have led them to reduce dose intensity in those patients. These factors were absolute neutrophil count nadir less than 500 in the first cycle, a drop in hemoblobin from baseline to the midcycle of the first cycle and in patients who had previously undergone radiation therapy.
Managing patients presenting with afebrile neutropenia
Managing patients who present with afebrile neutropenia is a challenge. A key issue is the threshold neutrophil count at which one feels comfortable treating. In my career, I’ve gravitated to using from 800 to 1,000 neutrophils as my cutpoint for either delaying or reducing dose.
Typically, I will delay treatment one to three days and repeat counts. I won’t delay a full week, which has historically been the “knee-jerk” reaction. Doxorubicin plus cyclophosphamide has a very abbreviated period of neutropenia. It can go quite low, but usually it’s not very prolonged, which is probably why these women don’t have a very high risk of febrile neutropenia.
In patients with high-risk disease, I do everything possible to avoid reducing their dose. Use of growth factors is an option. Another rational option is to forge ahead with therapy, especially with dose-dense therapy in which we’re automatically using growth factor support. I think we’re going to find that even women in the 21-day cycles are going to receive growth factors for the future cycles, and they’ll probably do fine.
I don’t use growth factors universally. I consider age and comorbidities, and if I think chemotherapy presents a real risk of future complications to the woman, I’ll add growth factors. In my experience, probably 25 to 30 percent of patients receive growth factor support at some point. I believe the more rational approach is to target it to the highest-risk group of patients and do it preemptively as opposed to waiting until they’re hospitalized or already neutropenic. Growth factors are much less effective once the patient is neutropenic.
Nonprotocol selection of adjuvant chemotherapy
In the nonprotocol setting — certainly in the younger node-positive population and even the younger elderly population — my colleagues and I often utilize an ACT type of regimen. We are still waiting for data to mature, but many of us are convinced that the taxanes add to the long-term outcomes in those patients; until proven otherwise, we believe patients should have the benefit of the doubt and be offered the taxane.
In patients with node-negative disease, I typically utilize AC every three weeks. However, patients and family members are inquiring about dose-dense schedules. Generally, I will comply with their desire for dose-dense AC because that regimen does not appear to be any more toxic than conventional scheduling. Additionally, I am willing to accept that the node-positive data will probably extrapolate, at smaller increments of benefit, to the node-negative population. If a woman asks for the dose-dense approach, I don’t have a compelling reason to refuse.
Select publications
|
| Dr Lyman is a Professor of Medicine and Oncology at the University of Rochester School of Medicine and Dentistry and Director of Health Services and Outcomes Research of the James P Wilmot Cancer Center at the University of Rochester Medical Center in Rochester, New York. |
|
