| You are here: Home: BCU 8|2004: G Thomas Budd, MD
G Thomas Budd, MD |
EDITED COMMENTS |
 Multigene RT-PCR assay for predicting recurrence in patients with node-negative breast cancer Multigene RT-PCR assay for predicting recurrence in patients with node-negative breast cancer
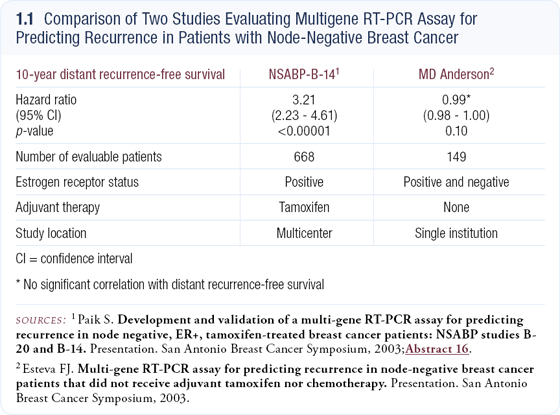
At the 2003 San Antonio Breast Cancer Symposium, Dr Soon Paik presented validation data from NSABP-B-14 demonstrating that a new multigene RT-PCR assay could identify gene expression profiles predictive of recurrence in patients with node-negative, ER-positive breast cancer who previously received adjuvant tamoxifen.
On multivariate analysis, this assay was a significantly more powerful predictor than other conventional clinical features. On the other hand, at the same meeting, Dr Esteva presented data from an MD Anderson trial in which the same assay did not fare so well. Esteva’s data examined a more diverse group of patients who had not received any adjuvant therapy (1.1).
I find the Paik NSABP data compelling. It has the advantages of being a multicenter trial conducted throughout the United States in both academic centers and community oncology practices, and the blocks were collected prospectively over a defined treatment period.
The data demonstrate the value of this assay in patients treated with tamoxifen. Presumably these data can be generalized to patients treated with aromatase inhibitors; however, that has not been demonstrated.
The Program for the Assessment of Clinical Cancer Tests (PACCT) is planning to study this new technology. The simplest way to validate it would be to study it prospectively, but that would take years to accomplish and by the time the study was completed, newer technology would be available.
Another possibility is to prospectively study whether this, or a similar assay, can be used to select patients at low risk who can be spared chemotherapy, or patients at high risk who need intensive chemotherapy. Clearly, multiple approaches need to be considered, and the final trial design is still being developed.
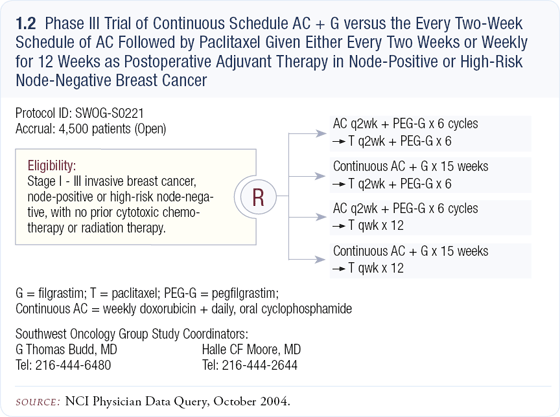
SWOG-S0221: Dose-dense versus continuous adjuvant chemotherapy
In SWOG-S0221, AC is administered in either an every two-week dose-dense manner with pegfilgrastim versus what might be described as a metronomic schedule with filgrastim. Both schedules are then followed by paclitaxel (1.2). We chose six cycles of AC and paclitaxel in the control arms for several reasons. First, by imposing similar durations of treatment in all arms, we avoid wondering later whether an inferior outcome in any arm reflected the duration of treatment.
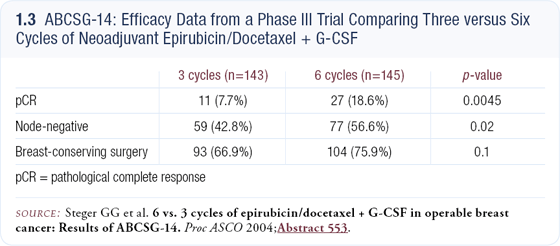
In addition, some data suggest six cycles is superior, although this is still controversial. An Austrian trial presented at ASCO in 2004 compared three cycles to six cycles of preoperative epirubicin/docetaxel, and the six-cycle schedule was more efficacious (1.3). Older trials with CMF have had mixed results — some show equivalence while others show six cycles might be better.
In the SWOG study arms, weekly doxorubicin and daily cyclophosphamide are given for 15 weeks. In preclinical models examining the antiangiogenic effects of chemotherapy, it appears that frequent administration of low-dose chemotherapy may be superior to the maximum tolerated dose (MTD) model.
In addition, this more continuous schedule may provide a good chemotherapy base upon which to add other antiangiogenic approaches. Evidence indicates that with the MTD schedule, a burst of vasculogenesis occurs between cycles and possibly hematopoietic growth factors augment that, but it is unclear whether that occurs with weekly doxorubicin and daily cyclophosphamide.
Dose-dense chemotherapy
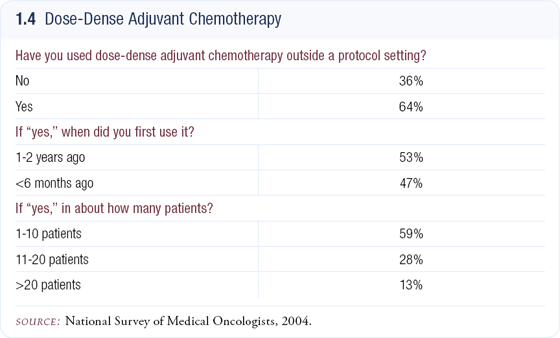
When I use adjuvant AC followed by paclitaxel, I employ a dose-dense schedule because dose density has been demonstrated to be superior; however, the clinicians not employing dose density may feel it needs further follow-up (1.4). In examining the data from CALGB-9741, it is possible that the benefits of dose density pertain only to the paclitaxel treatment, and it may not be advantageous for the anthracycline-based portion of the regimen.
Data shows that paclitaxel given more frequently than every three weeks is superior, whereas the GONO-MIG1 trial, which compared six cycles of 5-FU/ epirubicin/cyclophosphamide given every two weeks versus every three weeks, failed to demonstrate a convincing difference between those two schedules.
Intergroup trial of chemotherapy in patients with node-negative tumors
In this current design, dose-dense doxorubicin/cyclophosphamide is given every two weeks for either four or six cycles, and this is compared to dose-dense paclitaxel given every two weeks for four or six cycles. This a very clean study that will help look at this issue of six versus four cycles and will directly compare an anthracycline and a taxane.
Moreover, it dovetails very nicely with SO221 that uses six cycles of dose-dense doxorubicin/cyclophosphamide and six cycles of dose-dense paclitaxel in the control arms.
Pegfilgrastim in dose-dense adjuvant chemotherapy
No major problems have been reported using pegfilgrastim in dose-dense AC 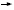 T, and we are using it in SWOG-S0221 in the every two-week arms because of the ease of administration. Pegfilgrastim has been used in patients with lymphoma receiving CHOP every two weeks and patients with Hodgkin’s disease using doxorubicin, bleomycin, vinblastine and dacarbazine every two weeks, so I believe we have sufficient data to justify its use, at least in the anthracycline phase of the trial. T, and we are using it in SWOG-S0221 in the every two-week arms because of the ease of administration. Pegfilgrastim has been used in patients with lymphoma receiving CHOP every two weeks and patients with Hodgkin’s disease using doxorubicin, bleomycin, vinblastine and dacarbazine every two weeks, so I believe we have sufficient data to justify its use, at least in the anthracycline phase of the trial.
When using pegfilgrastim with AC followed by paclitaxel, patients may present for paclitaxel treatment with relatively high white counts; however, I have found that simply proceeding with therapy and continuing pegfilgrastim has been safe and well tolerated.
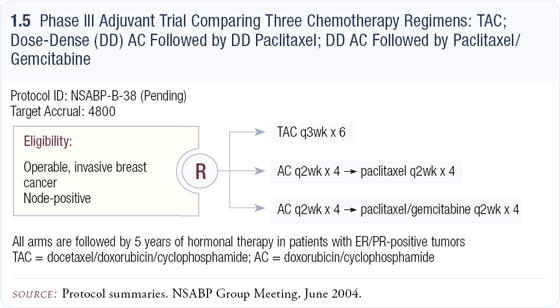
NSABP-B-38: Adjuvant chemotherapy
NSABP’s pending study, B-38, proposes to compare the two optimal anthracycline/ taxane regimens with a new combination in the paclitaxel phase (1.5). It’s a good trial design because in addition to determining whether one of the two standard combinations is superior, it examines an agent new to the adjuvant setting — gemcitabine.
At ASCO, Kathy Albain reported on a trial that showed an advantage for gemcitabine/paclitaxel versus paclitaxel alone in patients with metastatic disease. While the every two-week schedule is a bit of a leap, it was necessary to make it comparable to the dose-dense paclitaxel schedule.
Combination versus single-agent chemotherapy for metastatic disease
In the metastatic setting, I generally use sequential single agents rather than combination therapy, except in situations in which an early response is vital, such as lymphangitic pulmonary disease. The sequence I utilize depends on the patient’s prior therapy, comorbid conditions and lifestyle, so it’s extremely variable. I usually use a taxane, which reflects the fact that most of the patients who are relapsing now have not previously been treated with a taxane.
I believe docetaxel is superior to paclitaxel, so for a younger or more seriously ill patient, I tend to use docetaxel every three weeks. In an older patient, I prefer weekly paclitaxel. If a patient has received a taxane and progresses, I generally use capecitabine, starting at two grams per meter squared per day for two weeks, then one week off. Some patients do fine, but some develop a toxicity during the second week, so I shorten the duration of treatment with subsequent cycles.
I have also become more liberal with combination therapy and if a patient is quite ill, I generally use capecitabine/docetaxel. Paclitaxel/gemcitabine is less toxic; however, in examining the various trials, it appears capecitabine/docetaxel may be superior in terms of survival.
Docetaxel has a survival advantage over paclitaxel; paclitaxel plus gemcitabine has a survival advantage over paclitaxel alone; and docetaxel plus capecitabine has been shown to be superior to docetaxel. Obviously, it’s an indirect comparison, but in my experience, the majority of patients I have treated with capecitabine/docetaxel have benefited, although they have also experienced significant toxicity.
Adjuvant therapy for postmenopausal women with ER-positive tumors
In this setting, aromatase inhibitors are superior to tamoxifen. The ATAC trial demonstrated a reduced recurrence rate and event rate with aromatase inhibitors. Various other trials switching at two or three years also showed aromatase inhibitors to be superior.
Some argue that starting adjuvant hormonal therapy with tamoxifen is superior biologically; however, if you start with tamoxifen to set the tumor up for aromatase inhibition and estrogen withdrawal, a patient who may not have relapsed on an aromatase inhibitor may relapse while on tamoxifen.
I believe that aromatase inhibitors should be used initially in most patients. I use anastrozole because that has been studied, but as more trials mature, I suspect we’ll find any one of these agents can be used up front. I’m relatively certain they will all prove to be superior to tamoxifen.
Fulvestrant in the metastatic setting
SWOG is conducting a trial in metastatic disease comparing anastrozole alone to anastrozole plus fulvestrant. Preclinical rationale supports this and if an advantage is seen with the combination, then it would be logical to study it in the adjuvant setting. Clearly fulvestrant does not have the estrogenic effects of tamoxifen. It appears to be equivalent to anastrozole but it may be less appealing to study in the adjuvant setting because it involves a monthly injection.
Select publications
 |
Dr Budd is Director of Medical Oncology in the Breast Cancer Program and Director of the Cancer Center Chemoprevention Program at The Cleveland Clinic Foundation’s Hematology and Medical Oncology/Taussing Cancer Center in Cleveland, Ohio. |
|
