|
You are here: Home: BCU 8|2004: Daniel R Budman, MD
 |
Adjuvant Chemotherapy in the Elderly —
Current Options and Research Protocols |
 |
Daniel R Budman, MD
Professor of Medicine
New York University
North Shore University Hospital |
| 4.2 |
|
| |
|
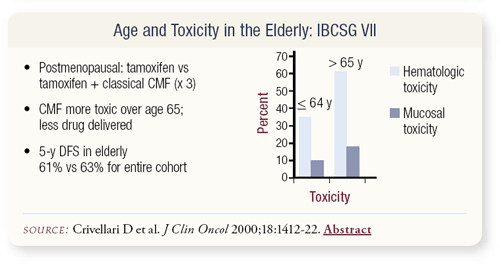
SLIDES 4.1 , 4.2 Data from the International Breast Cancer Study Group, published in the Journal of Clinical Oncology, evaluate the toxicity of chemotherapy in elderly patients. In postmenopausal patients, the initial focus was to look at endocrine therapy versus chemoendocrine therapy — tamoxifen versus tamoxifen plus classical CMF.
CMF was more toxic in the older age group, in terms of both hematologic and mucosal toxicity, and less drug was delivered. A lot of agents have a threshold effect, and if we cut the dose, we’re diminishing efficacy with most of the drugs we use. This led the CALGB Breast Committee to consider what should be done with the elderly patient population.
|
| |
|
|
| 4.3 |
|
| |
|
|
| |
|
 |
| 4.5 |
|
| |
|
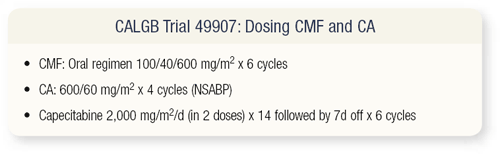
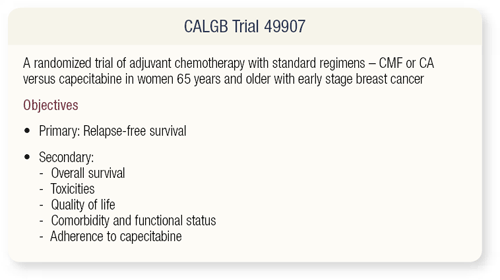
SLIDES 4.3 - 4.5 CALGB trial 49907 asks, Can we offer benefit to elderly patients with some of the newer chemotherapy agents? We have numerous agents available. As patients get older, they have comorbid conditions and can’t as easily get back and forth to the office for treatment. Ideally, it would be nice to have an oral agent. This trial was discussed by the CALGB Breast Committee for some time and was felt to have merit. Hyman Muss advocated for this trial and initially met a lot of resistance because people asked, “How can you not give IV chemotherapy?”
The reason is because oral agents are active, and we’re looking for efficacy in the adjuvant setting. This study is ongoing and is available through the CTSU or the Cooperative Group mechanism. It’s basically doctor’s choice — CMF or AC versus capecitabine — for women who are older than 65 years of age and have operable breast cancer.
The objectives are what one would expect: prevent relapse, extend overall survival and avoid toxicity. Quality of life, comorbidities and functional status are important secondary objectives.
Patients with operable disease are randomly assigned to six cycles of classical CMF with all the toxicities or classical AC or capecitabine for six cycles, given on days one through 14 on an every 21-day schedule. Obviously, patients who have hormone receptor-positive disease receive tamoxifen or an aromatase inhibitor.
|
| |
|
|
| 4.6 |
|
| |
|
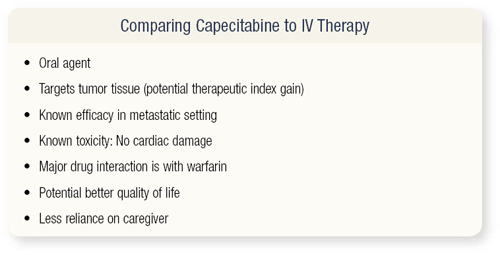
SLIDE 4.6 Why would the CALGB want to conduct this trial? Capecitabine has the advantage of oral administration and it targets tumor tissue. My major interest has been clinical pharmacology and drug development for the last 15 years, and this is an interesting drug because it’s changing the way we think in oncology.
We are trying to target tissue and diminish toxicity rather than just using an active drug. Capecitabine has known efficacy and doesn’t cause cardiac damage, which is a major issue as patients get older. The only major drug interaction is with warfarin.
|
| |
|
|
| 4.8 |
|
| |
|
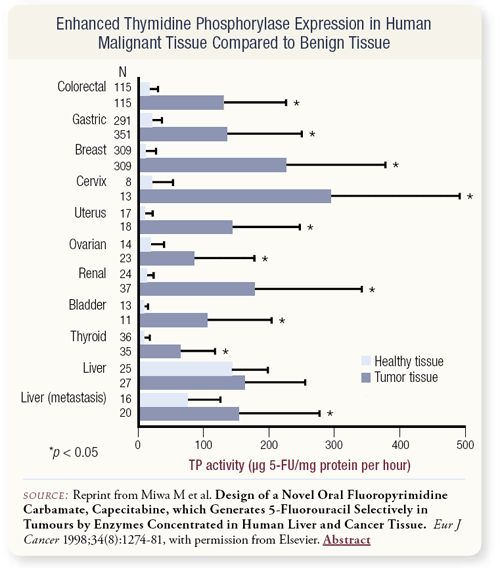
SLIDE 4.7 We also have issues of redundancy of pathways, or you can try to use the unique characteristic of the tumor itself. Other examples of targeted therapy include trastuzumab in patients with HER2-positive disease and the tyrosine kinase inhibitors that are being developed. Capecitabine was particularly attractive to us when we first started working with the drug. The enzyme thymidine phosphorylase, also known as platelet-derived growth factor, is overexpressed in 40 percent of breast cancers, so it’s a marker for aggressiveness and it’s a marker for some diseases, including breast cancer. Thymidine phosphorylase is necessary to activate capecitabine into the active metabolite, so it’s a method of targeting the tumor.
SLIDE 4.8 This slide demonstrates work that has evaluated thymidine phosphorylase in a variety of tumors, and in the majority of tumors, it’s overexpressed compared to healthy, normal tissue.
|
| |
|
|
| 4.9 |
|
| |
|
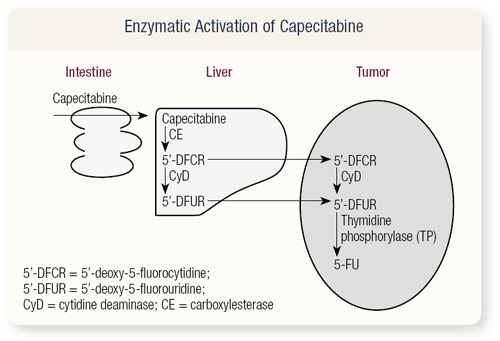
SLIDE 4.9 The mechanism of action of capecitabine is based on a drug called DFUR, which was developed in Japan. The problem with DFUR was that thymidine phosphorylase was in the gut, so if you took it in oral form you would get diarrhea. The chemists were very clever and put substitutions on it so it would not be activated in the gut — it has to be activated in the liver. It is then transferred to the tumor, where it undergoes metabolism from 5’-DFUR to 5-FU and concentrates in the tumor.
|
| |
|
|
| 4.10 |
|
| |
|
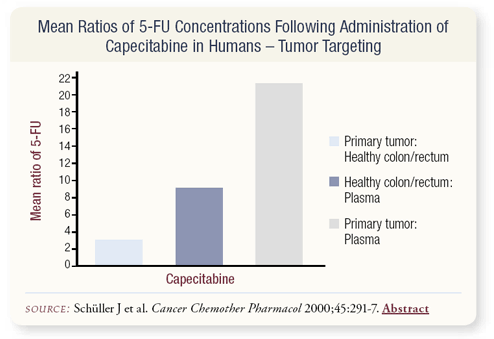
SLIDE 4.10 How do we know capecitabine increases the concentration of 5-FU in the tumor? Data have primarily been collected in colorectal cancer. Patients who were treated with a laparotomy were given capecitabine; then biopsies of the tumor and normal tissue were performed and concentrations of 5-FU were measured. A threefold greater concentration of active metabolite was present in the tumor compared to the surrounding normal tissue, indicating that capecitabine does, indeed, target the tumor.
|
| |
|
|
| 4.11 |
|
| |
|
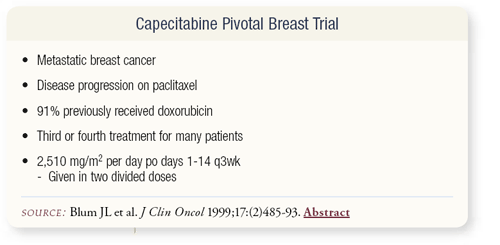
SLIDE 4.11 This led to a pivotal breast trial led by Joanne Blum in patients with metastatic breast cancer who progressed on paclitaxel. Most of them were previously treated with anthracyclines, and many underwent two or three different treatments. They were administered a dose of capecitabine that I think is a little too high — 2,510 mg/m2 given in two divided doses on days one through 14 every three weeks.
|
| |
|
|
| 4.12 |
|
| |
|
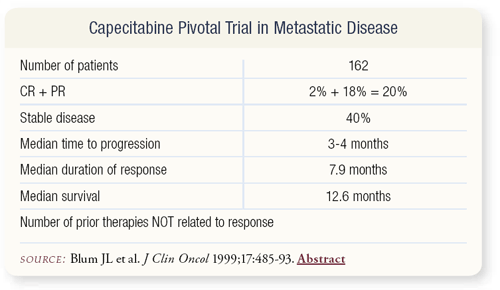
SLIDE 4.12 In these heavily pretreated patients, capecitabine resulted in a 20 percent response rate and increased disease stability.
|
| |
|
|
| 4.13 |
|
| |
|
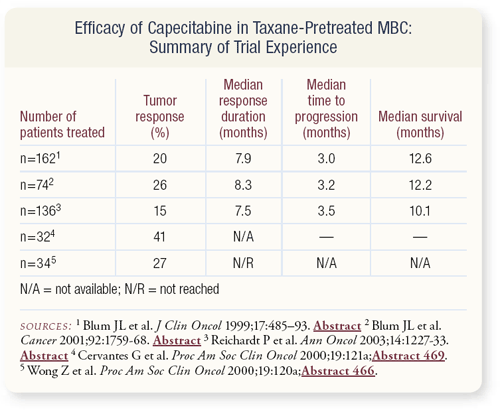
SLIDE 4.13 The efficacy of capecitabine has been confirmed in a variety of other studies. We have several hundred patients with taxane-pretreated metastatic disease who had demonstrable benefit from capecitabine. The CALGB Breast Committee considered it necessary to show that, if we’re going to randomly assign patients to another drug, capecitabine is a reasonable choice.
|
| |
|
|
| 4.14 |
|
| |
|
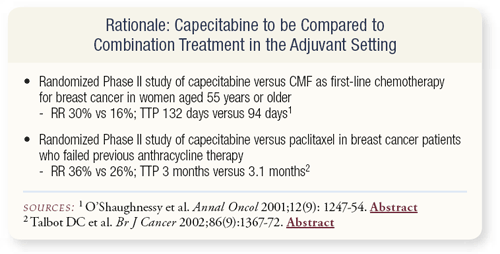
SLIDE 4.14 With which agents should capecitabine be compared? An underpowered randomized Phase II trial compared capecitabine to CMF. An equivalence study would require thousands of patients, but the efficacy seems to be in the same ballpark. If anything, capecitabine may be slightly better than CMF or paclitaxel administered every three weeks.
|
| |
|
|
| 4.15 |
|
| |
|
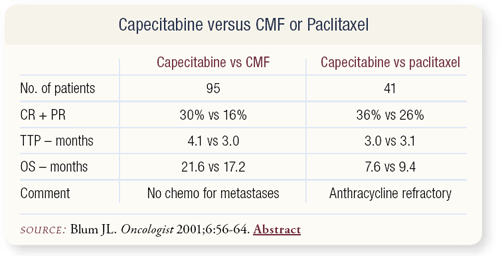
SLIDE 4.15 This is the same data presented a different way. In both small Phase II studies, the response rate was 30 percent or more and the time to progression also was similar.
|
| |
|
|
Additional ways exist to exploit tumor targeting
| 4.16 |
|
| |
|
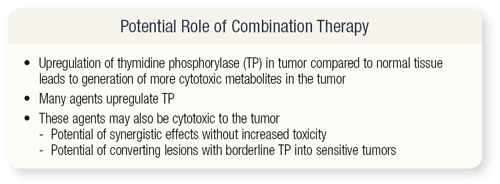
SLIDE 4.16 With combination chemotherapy, if one could enhance the target, then obviously you would expect more antitumor activity. Many cytotoxic agents actually enhance and upregulate thymidine phosphorylase. These agents can also be cytotoxic, so you can potentially achieve a synergistic effect and upregulation of TP.
|
| |
|
|
| 4.17 |
|
| |
|

SLIDE 4.17 Andy Seidman’s data and the ECOG Trial 1193 chaired by George Sledge suggest that using drugs in sequence in metastatic breast cancer may be just as good as combination therapy.
|
| |
|
|
| 4.18 |
|
| |
|
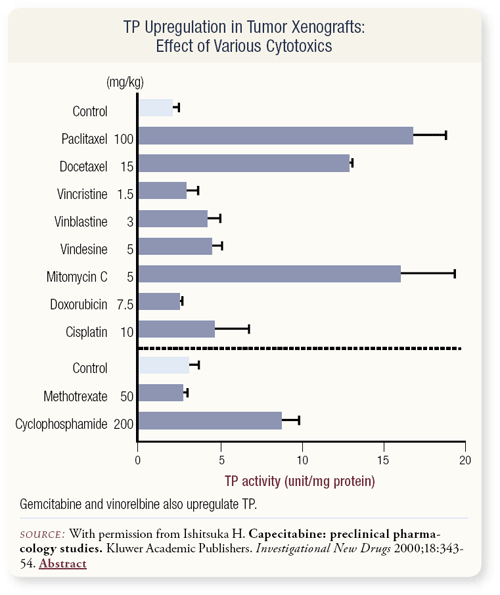
SLIDE 4.18 Preclinical data demonstrate that a variety of drugs — particularly the taxanes and cyclophosphamide — can upregulate thymidine phosphorylase.
|
| |
|
|
| 4.19 |
|
| |
|
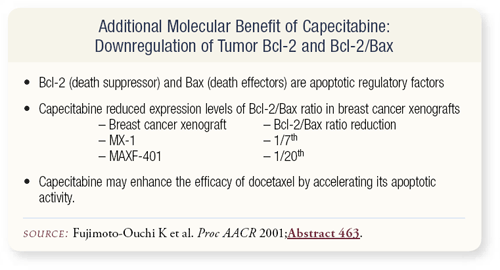
SLIDE 4.19 Another issue that’s come out more recently is that capecitabine is not 5-FU. In contrast to 5-FU, capecitabine seems to affect tumor Bcl-2 and Bcl-2/Bax combinations, so capecitabine becomes even more intriguing.
|
| |
|
|
| 4.20 |
|
| |
|
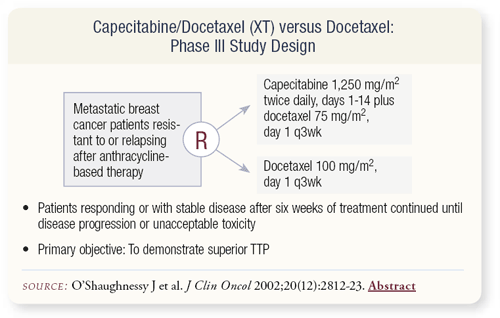
SLIDES 4.20 In preclinical work, capecitabine doesn’t look like 5-FU. If you use a combination of docetaxel and capecitabine a marked antitumor effect can be achieved, which is not seen with 5-FU. This preclinical data led Joyce O’Shaughnessy to perform a Phase III clinical trial comparing the combination of capecitabine and docetaxel (XT) to docetaxel alone in heavily pretreated patients with metastatic breast cancer.
|
| |
|
|
| 4.22 |
|
| |
|
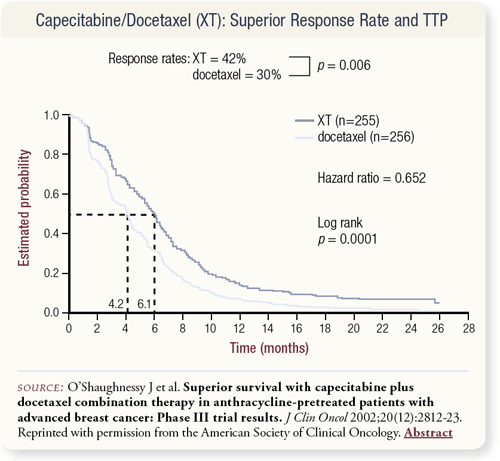
SLIDES 4.21, 4.22 The response rate was higher with the combination, and this is where the study becomes interesting.
The capecitabine/docetaxel combination had an early benefit for response rate and survival. Relative to the combination, an early and marked drop-off occurred with single-agent docetaxel at 100 mg/m2, which is a good treatment. This suggests that we really have to think about categorizing patients according to high risk and low risk. In a patient at high risk with a lot of visceral disease, many oncologists will utilize combination chemotherapy, but for a patient at low risk, we will consider sequential single agents.
|
| |
|
|
| 4.24 |
|
| |
|
SLIDES 4.23, 4.24 Currently, US Oncology is evaluating the capecitabine/docetaxel combination in the adjuvant setting.
For most of the drugs we’ve utilized, the “mantra” in oncology has been to “push the drug to the conventional limit.” If capecitabine concentrates in the tumor, we don’t have to push it hard. Unfortunately, the only information available from previous studies is that we don’t have a good dose-response curve. Again, this is retrospective data from the XT study.
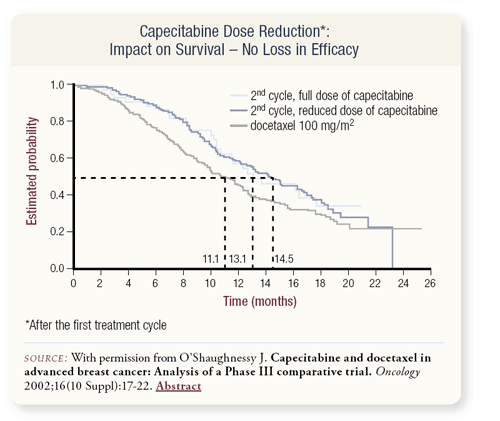
If the docetaxel dose in the XT arm is reduced by 50 percent, a significant decrease in antitumor activity occurs. This has also been demonstrated in prospective studies in which docetaxel 100 mg/m2 was better than 60 mg/m2 and 75 mg/m2, so docetaxel has a deep dose-response curve.
In comparison, if capecitabine is dose-reduced to 50 percent, no loss of efficacy is observed. The ability of capecitabine to concentrate in the tumor could explain this flat dose-response curve with capecitabine and may allow us to use doses that are not particularly toxic.
This graphically demonstrates that capecitabine dose-reduction did not diminish the survival benefit in the XT study.
|
| |
|
|
| 4.25 |
|
| |
|
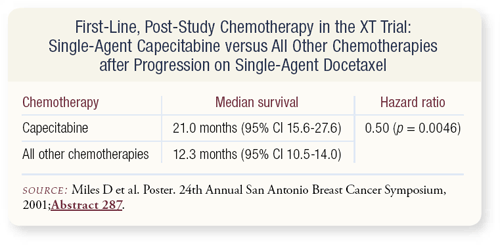
SLIDE 4.25 What happens to patients who progress while receiving docetaxel? Can we salvage them with another agent? Indeed, patients can be salvaged. Unfortunately, the XT trial did not have a mandated crossover, but crossing over to capecitabine after failing docetaxel can extend the life of some of these patients, suggesting that some patients should be treated with single-agent therapy.
What about using combinations of targeted agents?
|
| |
|
|
| 4.26 |
|
| |
|
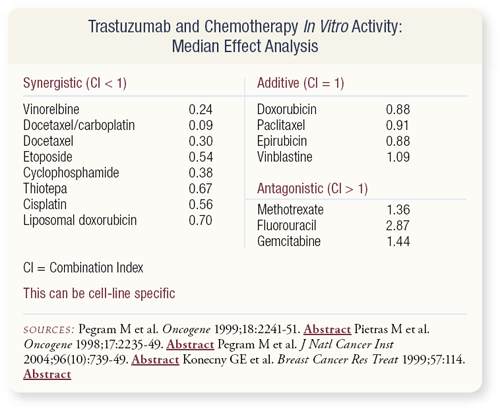
SLIDE 4.26 Mark Pegram and the group at UCLA, as well as other investigators have utilized an evaluative in vitro approach called median effect analysis, whereby tissue culture is evaluated for synergism or antagonism. Median effect analysis doesn’t inform us about the therapeutic index but it tells us whether or not the drugs are antagonistic. If the drugs are antagonistic, should we be administering them in combination?
Controversy always exists with models. This data depicts trastuzumab in combination with various agents in a cell line that overexpresses HER2. This data was the basis for combining the platinums and taxanes with trastuzumab. In this case, the fluoropyrimidines were antagonistic, but one of the difficulties with this type of model system is it can be cell-line specific.
|
| |
|
|
| 4.27 |
|
| |
|
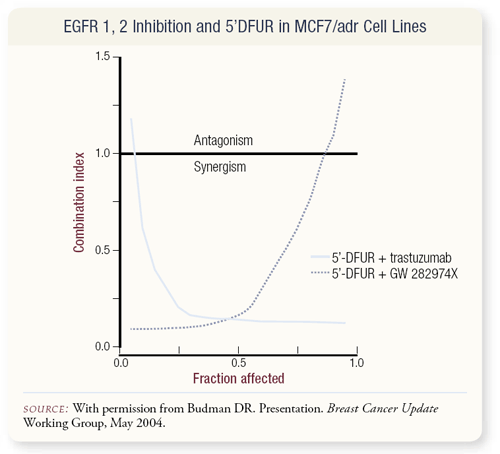
SLIDE 4.27 At the 2002 San Antonio Breast Cancer Symposium, we presented data evaluating the metabolite of capecitabine, 5’DFUR, with either a dual kinase inhibitor GW 282974X (provided by GlaxoSmithKline) or trastuzumab using the median effect in vitro assay technique. We continued these studies and will submit the full manuscript for publication shortly. Depending upon the breast cancer cell line studied, 5’DFUR combined with trastuzumab could demonstrate either synergistic or antagonistic cytotoxic effects. The results of this assay are shown graphically in the slide in which a multiplyresistant breast cancer cell was studied in vitro with the drug combinations. Combination Index values less than 1 are synergistic and fraction affected indicates the degree of cytotoxicity induced by the combination of drugs at various concentrations.
The model is most accurate at a fraction affected value of 0.5. For this cell line under the culture conditions we employed, the combination of 5’DFUR with trastuzumab is synergistic. As discussed previously, other investigators have noted antagonism between fluoropyrimidines and trastuzumab in cell line model systems. Therefore, we have a clinical dilemma in that the effect of the combination of trastuzumab with 5’DFUR in a model system does not give a global answer whether or not this is a useful combination. There is additional data to suggest that this combination may be of value as Fujimoto-Ouchi and coworkers have examined a xenograft model of breast cancer in which the combination of 5’DFUR and trastuzumab were at least additive. Hence, ongoing studies of trastuzumab with capecitabine are necessary to define the role of this combination in the clinical treatment of breast cancer.
|
| |
|
|
| 4.28 |
|
| |
|
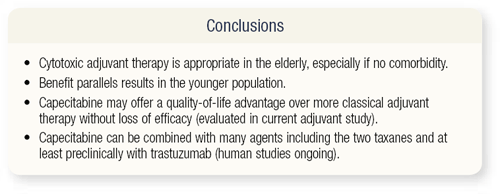
SLIDE 4.28 In conclusion, adjuvant cytotoxic chemotherapy is appropriate in elderly patients, but one must consider comorbidities. I urge physicians to consider enrolling patients in CALGB trial 49907, in which doctors choose AC or CMF versus capecitabine. If capecitabine turns out to be equivalent, then we have a kinder, gentler way of treating patients. Capecitabine can also be combined with numerous other chemotherapy and targeted agents, and these approaches are being evaluated in adjuvant trials.
|
| |
|
|
Select publications
|
|
|
|
