| You are here: Home: BCU 8|2004: Matthew J Ellis, MB, PhD, FRCP
Matthew J Ellis, MB, PhD, FRCP |
EDITED COMMENTS |
 Multigene RT-PCR assay for predicting recurrence in patients with node-negative breast cancer Multigene RT-PCR assay for predicting recurrence in patients with node-negative breast cancer
Previously, gene expression profiling required frozen material; however, perhaps less than one percent of breast cancers are stored in that fashion. A breakthrough came when it was discovered that the RNA wasn’t missing in a paraffin block, it was just fragmented. As a result of this discovery and other new technologies, a multigene assay was developed that is predictive of breast cancer recurrence despite adjuvant tamoxifen therapy. This assay was validated in the NSABP studies B-20 and B-14.
As a result, we now have a predictor that scores a woman’s risk of relapse between one and 100. Apparently, it is as powerful as tumor grade in predictive ability, but the assay is reproducible and grade is not. At first glance the ability to score a patient’s risk appears advantageous. On reflection, however, we know that each individual really has either a zero or a 100 percent risk — either they don’t relapse or they do.
The ideal predictor would be a black-and-white test that tells us which patients will relapse. This would be particularly useful when treating healthy, postmenopausal patients with node-negative, ER-positive breast cancer, in whom we’re considering adjuvant chemotherapy. Currently we administer a good deal of random therapy, which is expensive and results in unnecessary treatments and toxicities.
We need individualized management strategies that target the tumor’s biology and the patient’s risk. I’ve recently been involved with the Program for the Assessment of Clinical Cancer Tests, and we are working on a variety of approaches to prospectively test the new risk predictors. We are planning to conduct a large national trial in which we will use an initial version of a genebased classifier to group participants.
The trial design may be that patients at low risk will receive no adjuvant therapy, patients at high risk will receive adjuvant chemotherapy, and the patients in the gray area of risk will be randomly assigned to chemotherapy or no chemotherapy. We would then have a molecular snapshot of all the patients so that we could study the failures and build in new genes and new models to move closer to the optimal, black-and-white predictive version.
Tamoxifen versus aromatase inhibitors in the adjuvant setting
When the initial ATAC results were presented, I felt very positive regarding the data. This trial provided early evidence that aromatase inhibitors are more effective than tamoxifen. In addition, the aromatase inhibitors did not cause the serious, albeit not very common, toxicities of tamoxifen — namely endometrial cancer and stroke. Weighing the risks and benefits of these two therapies is what motivates me to prescribe aromatase inhibitors for adjuvant therapy in postmenopausal women with ER-positive breast cancer.
Some clinicians continue to argue that tamoxifen is the standard of care, given the amount of data and length of follow-up we have with that agent, and I respect that opinion. In addition, the effectiveness of aromatase inhibitors in the late adjuvant setting could strengthen the argument to begin endocrine therapy with tamoxifen. However, that argument focuses on patients who made it to five years without a relapse, whereas the ATAC data focuses on the first five years, so I continue to favor the aromatase inhibitors.
I certainly understand the viewpoint of the ASCO Technology Assessment. There’s a genuine concern that if we proclaim that we have the final answer in the debate between tamoxifen and aromatase inhibitors in the adjuvant setting, it could kill some very important clinical trials. It’s better to acknowledge that it’s an ongoing open question. While I believe that is reasonable, making such a statement in a large forum where you need to respect many opinions is much different than making a treatment decision for the patient sitting in front of you.
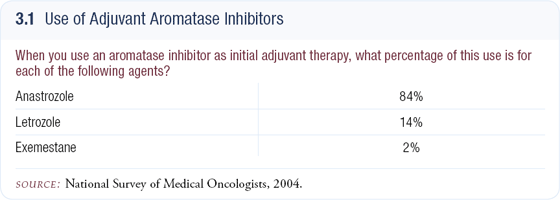
Initially I individualized first-line adjuvant endocrine therapy based on patient variables, but that changed as the cumulative data were reported. Now I generally prescribe aromatase inhibitors for all eligible postmenopausal patients up front. As for which aromatase inhibitor to use, I believe most clinicians treat according to the available data, and that’s appropriate. At initial diagnosis, I prescribe anastrozole (3.1).
Endocrine therapy after five years of adjuvant tamoxifen
Whether to continue adjuvant hormonal therapy after five years of tamoxifen is a risk-based decision. While a patient with node-positive disease may benefit significantly, in a patient with node-negative disease and a tumor less than one centimeter in size, the relapse rate might only be a few percent — and reducing that risk with letrozole might not be a meaningful exercise.
The relapse data indicate that approximately half of the relapses occur before five years and the remaining 50 percent occur after five years. If a patient has a 20 percent risk at baseline and doesn’t relapse in the first five years, then at five years her relapse rate is 10 percent. In such a patient, letrozole may have a 40 percent effect in reducing the relapse rate, so the absolute benefit is approximately four percent.
That is similar to the effect we expect from adjuvant chemotherapy in patients at low risk, so I believe it’s a reasonable therapy. Also, the risks associated with letrozole in this setting appear to be modest, so it makes sense even for relatively low-risk patients. However, there are certainly patients in whom it makes no sense — perhaps patients with a relapse risk rate of five percent or less after five years.
As for the patient who completed her five years of adjuvant tamoxifen therapy a year or more ago, the risk of recurrence is fairly evenly spread over the next 10-year period. If she hasn’t relapsed already, you can do a back-of-an-envelope calculation to determine her residual risk.
Then the question is whether the late introduction of further adjuvant hormonal therapy would be helpful. We don’t really know. One could consider the patients who crossed over in the MA17 trial versus those who didn’t to gain a sense of whether it’s helpful, but that’s not randomized and may not be valid.
Neoadjuvant trials comparing tamoxifen and aromatase inhibitors
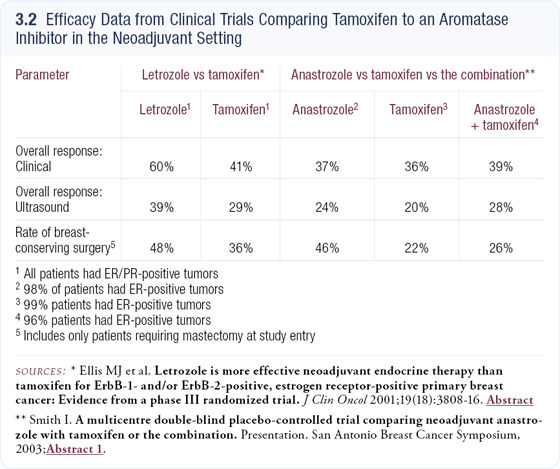
We conducted a neoadjuvant trial comparing letrozole to tamoxifen in postmenopausal women with ER-positive breast cancer. It was similar to the IMPACT trial in that they both compared tamoxifen and an aromatase inhibitor, and both trials are showing aromatase inhibitors to be clinically advantageous in favorably impacting the rates of breast-conserving surgery (3.2). The IMPACT trial studied anastrozole versus tamoxifen.
Also, our trial required that all patients be ineligible for breast-conserving surgery, so the tumors were large and the responses were easy to measure. However, the IMPACT trial enrolled some patients with smaller tumors, and when a tumor shrinks from two centimeters to one centimeter, clinically it’s difficult to be certain you’re truly measuring response. That might explain why the IMPACT study did not show much difference in clinical response between the arms.
In addition, the IMPACT trial had three arms — anastrozole, tamoxifen, and anastrozole plus tamoxifen — whereas our trial had only two arms, so their trial wasn’t as well powered to show a difference between just tamoxifen and an aromatase inhibitor. Mitch Dowsett examined proliferation at the cellular level in the IMPACT trial and reported that the proliferation changes appeared to be more profound in tumors treated with anastrozole than in tumors treated with tamoxifen.
We’re moving ahead with an ACOSOG neoadjuvant study comparing exemestane with or without celecoxib in postmenopausal women with ER-positive, Stage II/III breast cancer who are ineligible for breast-conserving surgery or whose tumors are inoperable. In the United Kingdom, Mike Dixon is the principal investigator for a trial comparing neoadjuvant letrozole and anastrozole. I believe it’s important to compare the various aromatase inhibitors because ultimately these agents will be off patent and inexpensive. Knowing which is the most efficacious will be important.
Select publications
 |
Dr Ellis is an Associate Professor of Medicine, Head of the Section of Medical Oncology, Director of the Breast Cancer Program and Co-Director of Translational and Clinical Research at Washington University School of Medicine. |
|
