| You are here: Home: BCU 9|2004: Gabriel N Hortobagyi, MD
Gabriel N Hortobagyi, MD |
EDITED COMMENTS |
 Randomized trial of neoadjuvant paclitaxel and FEC with or without trastuzumab in women with HER2- positive disease Randomized trial of neoadjuvant paclitaxel and FEC with or without trastuzumab in women with HER2- positive disease
Background
In the late 1990s, the adjuvant trastuzumab trials were being developed. My group had been working on neoadjuvant trials for the previous 15 to 20 years, so we wanted to evaluate trastuzumab-containing combinations in that setting. A substantial number of women had larger tumors when we developed this protocol, and we wanted to obtain biological information through multiple biopsies in the neoadjuvant setting.
Our initial proposal in 1998 followed shortly after the reports of cardiac toxicity associated with trastuzumab administered in combination with chemotherapy, especially the anthracyclines. We encountered a fair amount of resistance with respect to the use of an anthracycline with trastuzumab. We hypothesized — based on preclinical data from Dennis Slamon’s laboratory — that the temporal association between chemotherapy and trastuzumab was important to obtain maximal cytotoxicity and that the anthracyclines were clearly an important component of the treatment for women with HER2-positive tumors.
Trial design
Our trial (Buzdar 2004) was different from all others using trastuzumab either sequentially following the completion of adjuvant chemotherapy or in combination with chemotherapy regimens that excluded anthracyclines. We were criticized by our colleagues because we decided to pursue a trial in which trastuzumab was administered simultaneously with an anthracycline.
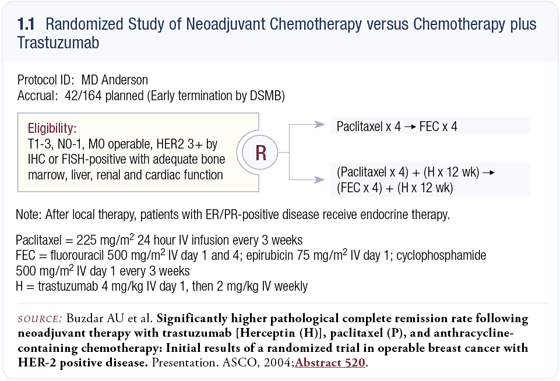
Our trial involved the best regimen at that time — every three-week paclitaxel followed by FAC; however, to minimize cardiac toxicity we elected to use epirubicin instead of doxorubicin. The neoadjuvant chemotherapy regimen was four cycles of paclitaxel followed by four cycles of FEC. Patients, mostly with T2 or T3 disease, were randomly assigned to receive neoadjuvant chemotherapy with or without simultaneous trastuzumab (1.1). Postoperative therapy was left to each investigator’s discretion.
The primary endpoint of the trial was the pathological complete response (pCR) rate, which was defined as no evidence of residual invasive disease either in the breast or the regional lymph nodes. Our trial was based on the assumption that the chemotherapy regimen would lead to a 21 percent pCR rate and that the addition of trastuzumab would improve the pCR rate by another 20 percent to 41 percent. Because of the potential for cardiac toxicity, we wanted to demonstrate a large difference. We believed a smaller difference would not be convincing enough to make this regimen generally accepted.
Trial results
We enrolled 42 patients over three years. We did not notice any adverse cardiac events during the trial, and as the trial went on we became more comfortable with the combination. Eventually, we began to see high pCR rates in our patients, and the Data Safety Monitoring Board (DSMB) was alerted to analyze the data. They recommended the randomization be discontinued, and a formal analysis was performed.
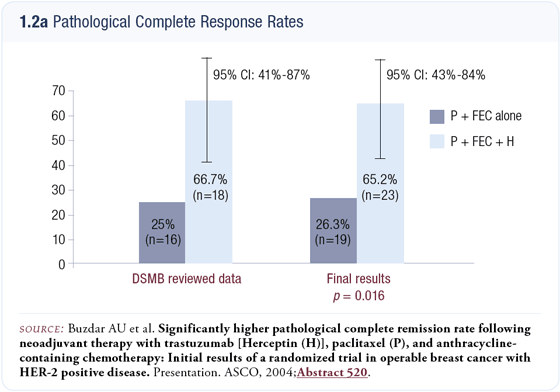
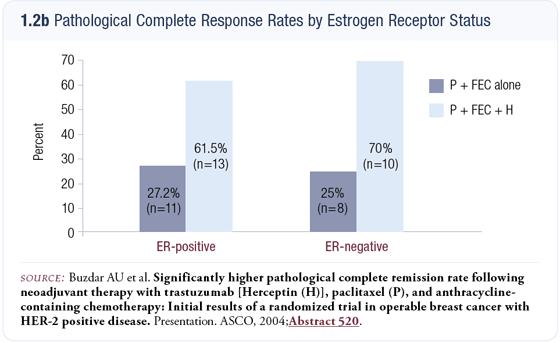
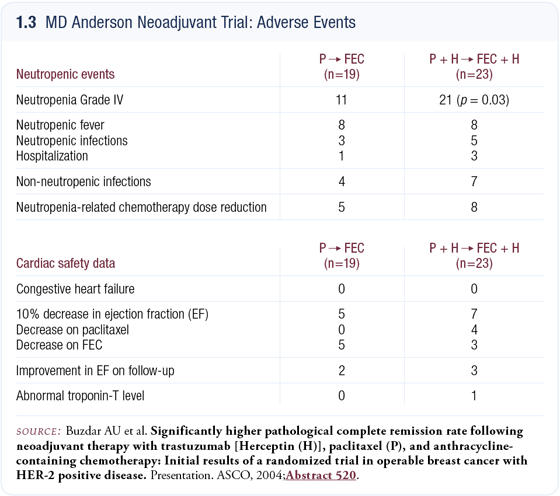
We exceeded the projected improvement in the pCR rate. In the subset of patients who had undergone surgery at the time of the DSMB’s analysis, the pCR rate was about 67 percent. After all 42 patients had undergone surgery, the data were similar except that the 95 percent confidence interval had narrowed (1.2 a and b). Long-term follow-up data are not yet available but we felt obligated to present the data and did so at the 2004 ASCO meeting.
We are now performing the correlative studies on the pretreatment and posttreatment biopsies from patients with residual disease. Ironically, the high pCR rate doesn’t leave any tissue; hence, the correlative studies are restricted to the pretreatment biopsies in those patients. In addition to the clinical examination for cardiac toxicity, we also monitored troponin levels and MUGA scans. In the entire group of patients, we did not see any episodes of congestive heart failure or cardiac-related deaths (1.3).
Nonprotocol use of neoadjuvant and adjuvant trastuzumab
Our results were very surprising, but very important. If you consider that a patient with a T3 tumor with palpable lymph nodes, to whom you administer adjuvant chemotherapy with trastuzumab, is a reasonable choice off protocol, why wouldn’t you do that if surgery was done up front and that same patient presents with 10 positive nodes de novo? All kinds of scientific and ethical questions arise about how to deal with this. Currently, we have a single-arm trial running to expand our database. We are considering opening that up for larger tumors, to include the locally advanced and inflammatory tumors, until additional trial concepts become available. We’d rather treat those patients within the safety of a clinical trial that requires extensive monitoring than treat them with adjvant trastuzumab off trial.
Nevertheless, we have agreed as a group that, if a patient with HER-2-positive primary breast cancer is considered for neoadjuvant chemotherapy and is not a candidate for, or does not agree to participate in, the ongoing trial, we would discuss and offer trastuzumab with the same regimen used in the study. Although we have discussed this extensively in my group, we have not extrapolated it to the adjuvant setting for the time being. It’s difficult to legislate what goes on between an individual patient and her physician, but as a group, we have agreed that it is generally not appropriate to start adjuvant trastuzumab outside of a clinical trial.
Randomized neoadjuvant trial comparing weekly paclitaxel and every three-week docetaxel plus capecitabine both followed by FEC
In a current trial, we are comparing two regimens — our previous best neoadjuvant regimen of weekly paclitaxel followed by FEC, and every three-week docetaxel plus capecitabine followed by FEC. Even though no direct comparisons have yet been reported, every three-week docetaxel is probably equivalent to weekly paclitaxel. One trial in patients with metastatic disease suggests that every three-week docetaxel is more effective, although more toxic, than every three-week paclitaxel (Jones 2003). On that basis, I believe the capecitabine plus docetaxel regimen will have greater efficacy than weekly paclitaxel.
Adjuvant aromatase inhibitors as initial therapy in postmenopausal women
Since the third generation aromatase inhibitors are better than tamoxifen, my postmenopausal patients with ER-positive disease who have not yet started adjuvant hormonal therapy will initially receive an adjuvant aromatase inhibitor — preferably anastrozole. We started to using adjuvant anastrozole instead of tamoxifen after the first presentation of the ATAC trial results.
Even if tamoxifen and anastrozole had been therapeutically equivalent, anastrozole would still be preferable because it was better tolerated. For us, the issue of osteopenia was always secondary. We already had experience with the bisphosphonates and monitoring patients for osteoporosis, because chemotherapy and ovarian ablation produce premature menopause and accelerated bone resorption. We felt quite comfortable in switching our front-line adjuvant therapy to anastrozole.
Aromatase inhibitors as crossover therapy in postmenopausal women treated with adjuvant tamoxifen
Our current practice in postmenopausal women who are taking adjuvant tamoxifen for any length of time is to switch to an aromatase inhibitor. We try to conform to the data from the randomized trials evaluating a crossover to the aromatase inhibitors, but taken together, those data appear to have a class effect.
A year ago, I would have said, “If the woman had received adjuvant tamoxifen for five years, I would switch to letrozole. If the woman had received adjuvant tamoxifen for two or three years, I would switch to exemestane.” Right now, I feel comfortable with any of the aromatase inhibitors at any point in time of switching. In addition to the MA17 trial with letrozole (Goss 2003) and the Intergroup Exemestane Study (Coombes 2004), the Italian trial (Boccardo 2003) with anastrozole reported similar results. In the absence of a head-to-head comparison, the toxicity profiles of these three drugs are very similar.
Aromatase inhibitors following five years of adjuvant tamoxifen
We base the decision to give aromatase inhibitors to women who have completed five years of adjuvant tamoxifen on the patient’s risk. In some patients the residual risk is so small that the benefit of additional therapy is marginal. Yet some of these patients have difficulty letting go of tamoxifen — it’s a safety net and they want to continue on adjuvant therapy. Others can’t wait to finish the treatment.
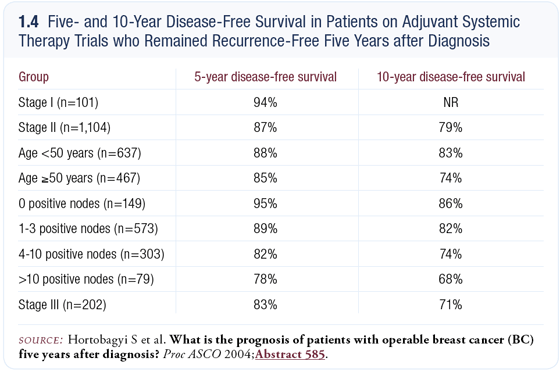
At ASCO 2004, I presented an abstract on the prognosis of patients with operable breast cancer five years after diagnosis (Hortobagyi 2004) (1.4). We pooled our adjuvant data dating back to 1974 from approximately 2,500 patients who had received adjuvant therapy and re-plotted the survivors’ disease-free survival five years after diagnosis.
We studied the pattern of relapse in the second five years and found that for most patients with Stage II and Stage III disease, the residual risk is sufficient to justify additional therapy, including patients with ER-positive tumors who received five years of tamoxifen.
If I believe a patient has a sufficiently high risk, I will consider offering an aromatase inhibitor six or even 18 months after she completed five years of tamoxifen. Where to draw the line is gray, because we don’t have good data on how to calculate residual risk at the end of five years of tamoxifen. We are rather proficient at calculating risk at the time of initial diagnosis by using Chuck Loprinzi’s model or Peter Ravdin’s Adjuvant! program, but we’re not very good at determining risk five years later.
Fulvestrant versus aromatase inhibitors in the metastatic setting
Assuming an aromatase inhibitor and fulvestrant are equivalent in efficacy, the choice of which agent to use may come down to patient preference. Some of my patients are perfectly happy with a monthly injection while others prefer an oral agent. For many patients, fulvestrant is financially favorable because of our arcane reimbursement system. We know that responses can be seen with either sequence — an aromatase inhibitor followed by fulvestrant or the opposite — but I believe it’s important we determine which is superior.
I believe the trials of fulvestrant underestimate the efficacy of this agent. The dosing schedule used was probably too low because by the time steady state was reached, many patients were off-study, presumably because of progression. In my group, we administer loading doses of 500 mg of fulvestrant, followed by 500 mg two weeks later and then 250 mg monthly.
The pharmacokinetics of fulvestrant suggest a loading dose would be beneficial, so it concerns me that the comparison of fulvestrant to anastrozole in a tamoxifen-resistant population might not have revealed the true efficacy of fulvestrant (Robertson 2003). It showed fulvestrant to be at least as effective as anastrozole, but I expected it to be superior. We may need to repeat some of these studies with a more appropriate dosing schedule.
Select publications
 |
Dr Hortobagyi is Professor of Medicine, Nellie B Connally Chair in Breast Cancer and Chairman of Department of Breast Medical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. |
|
