| You are here: Home: BCU 9|2004: Kathy D Miller, MD
Kathy D Miller, MD |
EDITED COMMENTS |
 Clinical trials of bevacizumab in women with metastatic breast cancer Clinical trials of bevacizumab in women with metastatic breast cancer
I believe the differences in the trial results of bevacizumab in breast cancer (Miller 2002) and colon cancer (Hurwitz 2004) trials were attributable to where during the course of the disease patients were treated, not some inherent difference in the biology of the cancers.
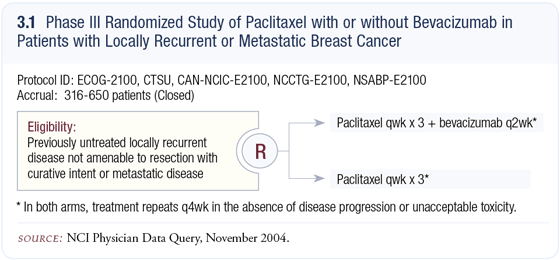
Our breast cancer ECOG trial evaluating bevacizumab with capecitabine enrolled patients with very advanced disease that was refractory to anthracycline and taxane therapy. Those patients could have received up to two other chemotherapy regimens for metastatic disease if they had received both an anthracycline and a taxane as adjuvant therapy (Miller 2002).
Dr Hurwitz’s trial of bevacizumab with IFL was conducted in patients with metastatic colon cancer who had not received previous chemotherapy for metastatic disease but could have had adjuvant chemotherapy (Hurwitz 2004). Likewise, our ECOG-2100 breast cancer trial enrolled patients with breast cancer who had not received chemotherapy for metastatic disease but could have had adjuvant chemotherapy.
Patients were randomly assigned to weekly paclitaxel with or without bevacizumab. The primary endpoint for ECOG-2100 is time to progression (3.1). Hopefully, the first interim efficacy analysis will be available within the next six to eight months; the final efficacy analysis is probably at least a year and a half away.
Proposed ECOG pilot trial of adjuvant bevacizumab in women with breast cancer
Because the effect of bevacizumab is expected to be greater in the adjuvant setting, a pilot adjuvant bevacizumab trial in patients with breast cancer has been proposed through the Eastern Cooperative Oncology Group. As much as I would like to conduct a full-scale study, we’re not ready to launch a definitive 3,000-patient adjuvant trial until the results from ECOG-2100 are available.
The purpose of the pilot adjuvant bevacizumab trial is to determine: (1) whether patients will be able to take the drug long term; (2) whether patients will be willing to continue therapy, because bevacizumab is administered intravenously and in some patients requires antihypertensive therapy; (3) whether patients treated with adjuvant therapy can maintain target serum drug concentrations comparable to those achieved in patients with metastatic disease; and (4) cardiac safety.
In our randomized trial of capecitabine with or without bevacizumab, an increase in the number of patients with either heart failure or cardiomyopathy was seen in those treated with bevacizumab. The total number of events was extremely small and not significantly different. All of those patients previously received anthracycline therapy and many, though not all, had received left chest wall radiation.
Two other trials — one in patients with refractory leukemia and the other in patients with sarcoma — evaluated bevacizumab concurrent with an anthracycline. Those two trials also reported cases of congestive heart failure and cardiomyopathy; however, trials in patients with diseases for which anthracyclines are not typically used have not reported cardiomyopathy.
If our pilot adjuvant bevacizumab trial demonstrates an incidence of clinical congestive heart failure of 10 percent or more, we would not move ahead with a full-scale adjuvant trial. Patients in the pilot adjuvant bevacizumab trial will be randomly assigned to receive bevacizumab concurrent with dose-dense AC followed by paclitaxel or dose-dense AC followed by bevacizumab concurrent with paclitaxel. The duration of therapy with bevacizumab for both groups will be six months.
Neoadjuvant capecitabine/docetaxel trial
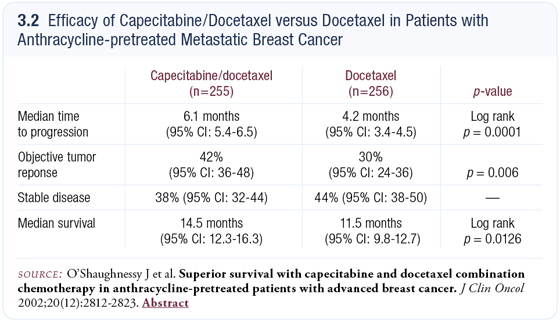
In one of our ongoing neoadjuvant studies, we’re trying to take advantage of genomics and proteomics to improve the individualization of therapy. The trial is based on the capecitabine/docetaxel (XT) regimen that Joyce O’Shaughnessy evaluated in the metastatic setting (3.2) (O’Shaughnessy 2002). For their first cycle of chemotherapy, patients will be randomly assigned to either capecitabine or docetaxel monotherapy. After that initial cycle, all patients will receive four cycles of both drugs in combination.
We’re collecting fresh tissue and a serum sample for serum proteomic analyses before the start of chemotherapy, after the first cycle of monotherapy and after the combination at the time of surgery. We are hopeful that the serum proteomics will be useful in predicting response because for many patients it is difficult to obtain a fresh tumor sample.
Investigators have predominantly evaluated the role of serum proteomics in identifying patients at risk of developing a malignancy or segregating patients with cancer from those with some benign condition. We’re trying to take proteomics a step further and determine if it will predict for response to individual therapies. We’re also performing tumor proteomic analyses in those patients. If we identify proteins in the serum that predict for response, we’ll also be able to determine whether those same proteins are actually in the tumor.
Sequential versus combination chemotherapy for women with metastatic disease
I am a confirmed “sequentialist.” In the metastatic setting, combination chemotherapy is appropriate for very few patients. The trials of the combination regimens of capecitabine/docetaxel (O’Shaughnessy 2002) and paclitaxel/ gemcitabine (Albain 2004) tell us that capecitabine and gemcitabine are active drugs in breast cancer and that patients with breast cancer are better off if they receive those drugs as part of their treatment. The trials, however, don’t tell us how to optimally use the drugs in the metastatic setting.
First-line therapy for a patient with asymptomatic ER-negative, HER2-negative metastatic disease
In my clinic, many patients with previously untreated, asymptomatic ERnegative, HER2-negative metastatic disease are treated first line with capecitabine monotherapy because it fits best with the goals of therapy. Capecitabine is by far the most convenient for patients. In my experience, it’s one of the most tolerable agents as chronic chemotherapy. Patients on capecitabine see me every nine or 12 weeks, and they don’t lose their hair.
Role of adjuvant aromatase inhibitors in postmenopausal women
Three aromatase inhibitors have been tested in the adjuvant setting in three different ways (Baum 2003; Coombes 2004; Goss 2003). Each of those three strategies demonstrated a decrease in early recurrences. We don’t have overall survival data yet because the trials aren’t mature enough. It’s possible that these strategies may not translate into an overall survival advantage because they were studied in postmenopausal elderly women who are more likely to die of other causes.
In a postmenopausal elderly woman, however, I’m not sure that overall survival is necessarily the most important outcome. If I can keep a 75-year-old patient from experiencing the physical pain and the emotional angst of a breast cancer recurrence before she dies of something else at age 79, I’ve done her well, even though I may not have altered her overall survival. I believe it will be important to obtain information from the long-term follow-up of those trials, including quality of life and the risk of heart disease and osteoporosis.
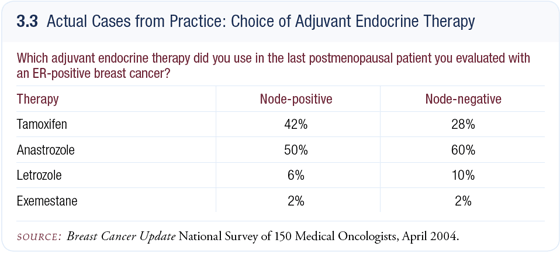
Of the postmenopausal women with newly diagnosed ER-positive early breast cancer in my practice, about 60 or 70 percent start on an adjuvant aromatase inhibitor, and about 30 or 40 percent start on adjuvant tamoxifen (3.3). If they’re starting on an adjuvant aromatase inhibitor, it’s always anastrozole because that’s the one for which we have data. I would use adjuvant tamoxifen for postmenopausal patients with low-risk breast cancer (eg, a Grade I, 1.2 cm tumor) who already have significant osteoporosis. In those patients, I worry that osteoporosis will have more of an impact than breast cancer on their overall health.
Extended adjuvant hormonal therapy with an aromatase inhibitor following five years of adjuvant tamoxifen
We talk about this to all of our patients who are finishing five years of adjuvant tamoxifen. Interestingly, one third of those women are concerned about stopping tamoxifen and would have badgered me to continue it. They believe additional therapy with an adjuvant aromatase inhibitor is the greatest thing and can’t wait to switch. Another third, I believe, have been taking their adjuvant tamoxifen to humor me, rather than because they believe they need it. They were counting the days until their therapy with tamoxifen was over and they are not interested in continuing therapy. The last third of the women are looking forward to completing therapy, but if an adjuvant aromatase inhibitor provides additional benefit, they want to consider it.
It’s a tough decision for many women, and we suggest they re-check their cholesterol and bone mineral density and consider those factors when making their decisions. I often suggest they stop taking tamoxifen and then see me in three months. I have them start taking the aromatase inhibitor about one month before they see me. This way, their personal experience with the toxicity can be considered in their decision-making.
Select publications
 |
Dr Miller is a Sheila D Ward Scholar of Medicine and Assistant Professor of Medicine in the Department of Hematology/Oncology at the Indiana University School of Medicine in Indianapolis, Indiana. |
|
