| You are here: Home: BCU 9|2004: Mark D Pegram, MD
Mark D Pegram, MD |
EDITED COMMENTS |
 Trial of neoadjuvant docetaxel, carboplatin and trastuzumab Trial of neoadjuvant docetaxel, carboplatin and trastuzumab
We are conducting a neoadjuvant study of docetaxel, carboplatin and trastuzumab in women with locally advanced breast cancer. Patients with HER2-negative disease receive docetaxel/carboplatin, while patients with HER2-positive disease are randomly assigned to docetaxel/carboplatin with or without trastuzumab.
Locally advanced breast cancer behaves more like metastatic than early-stage disease. Klaus Pantel’s work (Pantel 2004) suggests that these patients probably have many micrometastases, so I am more inclined to consider trastuzumab off-protocol in these cases or in patients with high-risk, Stage II HER2-positive disease.
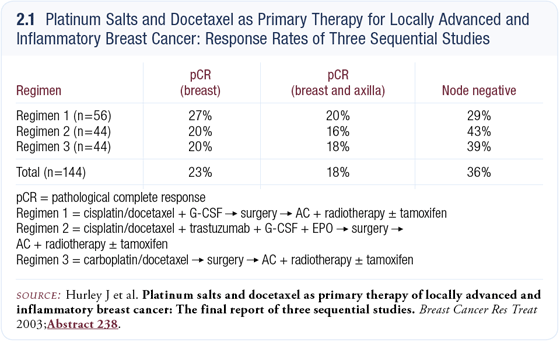
Our trial mimics Judith Hurley’s study at the University of Miami (Hurley 2003) and we have already seen impressive pathologic complete response rates similar to those reported by Dr Hurley (2.1). We are able to collect tissue before and after treatment, so a number of biochemical and molecular biologic correlates will be examined. The primary endpoints are molecular correlates of pathologic complete response.
Neoadjuvant chemotherapy with or without trastuzumab
I was impressed by the MD Anderson study examining trastuzumab, paclitaxel and anthracycline-containing chemotherapy in the neoadjuvant setting. The pCR rate in the trastuzumab-treated subjects was approximately 65 percent, which is extraordinary — in fact, it’s the highest I’ve ever seen in any primary breast cancer study (Buzdar 2004).
Although I believe the results are probably correct, a new standard of care has not been established and larger confirmatory trials are needed. The BCIRG adjuvant trastuzumab trial has accrued 3,150 FISH-positive cases and the European HERA study has accrued approximately 4,000 patients. We’ll have data from these studies and the North American Cooperative Group efforts in a couple of years.
Pathological complete response may prove to be a very important surrogate for future studies. Data from NSABP-B-27, being presented at the next San Antonio meeting, will tell us how well pCR correlates with long-term disease control. If indeed pCR proves to be a robust surrogate in the analysis of early-stage breast cancer, then the pCR data from the MD Anderson study may forecast the results of these larger adjuvant studies with regard to survival.
Rationale for combining trastuzumab and bevacizumab
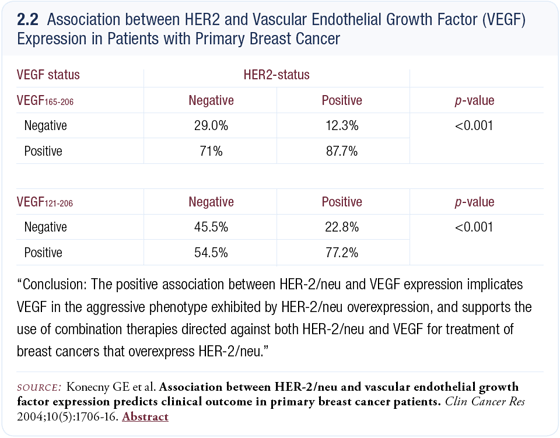
We conducted a study evaluating the effect of HER2 overexpression on VEGF expression in cell lines and found overexpression of HER2 yields an increase in VEGF expression. The next step was to determine the effect of HER2 overexpression in human tumors, so we examined 612 primary breast tumors and found the HER2-positive tumors were more likely to be VEGF-positive (2.2) (Konecny 2003).
When we evaluated clinical outcome, patients with tumors that had expression of both HER2 and VEGF had the worst prognosis. This data confirmed a connection between HER2 and VEGF in human subjects with primary breast cancer and provided one rationale for combining therapeutic approaches targeting both HER2 and VEGF.
Another rationale for combining these approaches involves the ability of VEGF to increase interstitial oncotic pressure within a solid tumor. Trastuzumab is a macromolecule unlikely to diffuse easily into a bulky solid tumor because of the high interstitial oncotic pressures.
However, treating that tumor with bevacizumab and reducing the VEGF levels could lower the interstitial oncotic pressure and potentially improve the delivery of trastuzumab and afford a better response.
Clinical response to bevacizumab in patients progressing on trastuzumab
We have seen anecdotal responses to bevacizumab in patients who are progressing on trastuzumab. Our experience mimics that of Sledge, Miller and Cobleigh in the Phase I trials of single-agent bevacizumab. These patients probably still have circulating trastuzumab even though a washout occurred since their last dose, and it may be that bevacizumab facilitates the penetration of trastuzumab into the tumors. We are conducting a Phase I/II trial evaluating bevacizumab and trastuzumab in patients with relapsed or metastatic breast cancer. If the results are favorable we will design a Phase III trial — possibly docetaxel, carboplatin and trastuzumab with or without bevacizumab in the metastatic setting.
Continuing trastuzumab at the time of disease progression
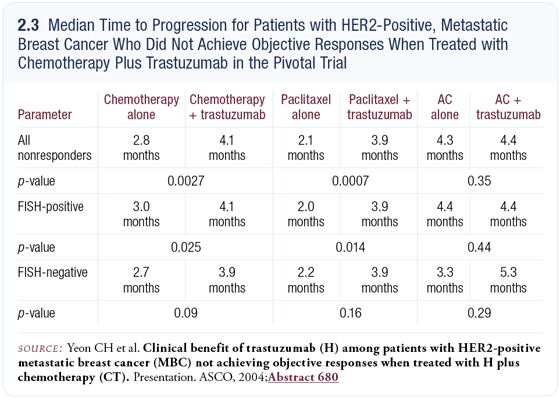
At ASCO 2004, Christina Haeyoung Yeon (Yeon 2004) presented some very provocative data developed at UCLA regarding the clinical benefit of trastuzumab among nonresponders in the pivotal trastuzumab trial (2.3). Oddly enough, the nonresponding subset of patients had a statistically significant longer time to progression than patients who received chemotherapy alone, suggesting continued biologic activity of trastuzumab even in the nonresponding subset. If indeed that’s the case, it reinforces the rationale for continuing trastuzumab beyond progression, especially if we can exploit other synergies with salvage cytotoxic agents.
The determination about when to discontinue trastuzumab is a clinical decision. I generally continue the agent in patients with a good performance status as long as they are not experiencing any long-term side effects and have adequate IV access, even though no randomized, controlled trials support continuation.
Trastuzumab in combination with hormonal therapy
In the Journal of the National Cancer Institute in 2003, we published a paper in collaboration with the Munich group showing a quantitative decrease in ER expression in over 900 patients with primary breast cancer when HER2 was amplified (Konecny 2003). Even in HER2-positive disease that was scored as ERpositive by IHC, quantitative measurement indicates a statistically significant lower level of ER than in HER2-nonamplified, ER-positive disease.
Therefore, inasmuch as the predicted response to any hormone manipulation is directly proportional to the abundance of the target, we would predict a priori that patients with ER-positive, HER2-positive disease would be less likely to respond to hormonal therapy than patients with ER-positive, HER2-negative disease.
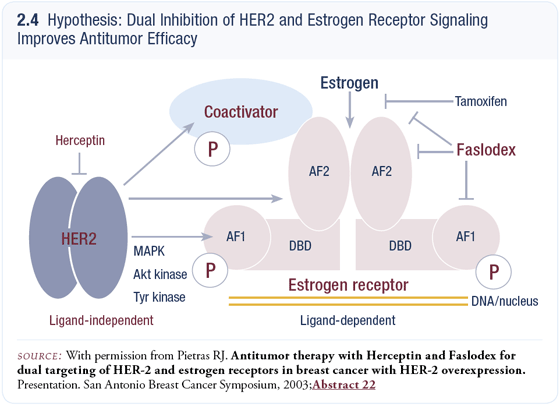
Randomized trials evaluating the strategy of combining hormonal therapy and trastuzumab are ongoing in Europe, including the study of anastrozole with or without trastuzumab, and some active Phase II nonrandomized studies, such as Matt Ellis’ trial of letrozole plus trastuzumab. We’ll have to wait for the data to determine the efficacy of combined receptor blockade, but it appears very promising in the preclinical models. We are about to launch a randomized clinical trial evaluating fulvestrant with or without trastuzumab based on the rationale that fulvestrant degrades the estrogen receptor and may eliminate any potential for cross talk between HER2 signaling pathways and the estrogen receptor (2.4) (Pietras 2004).
When treating patients with ER-positive, HER2-positive disease on first relapse, I generally begin with a combination of trastuzumab and chemotherapy. I have used trastuzumab and hormonal therapy in a nonprotocol setting; however, I always inform patients that the trials are ongoing and we have no data — although the emerging pattern appears promising. I believe we’ll see a survival advantage in first-line hormone-based regimens combined with trastuzumab in metastatic disease, as we have seen with first-line chemotherapy-based regimens combined with trastuzumab, and that we’ll find trastuzumab to be more active as first-line rather than salvage therapy.
Select publications
 |
Dr Pegram is Associate Professor of Medicine at the David Geffen School of Medicine at UCLA and Director of the Women’s Cancer Program-UCLA/Jonsson Comprehensive Cancer Center in Los Angeles, California. |
|
