Edward H Romond, MD |
EDITED COMMENTS |
 Combined analysis of NSABP-B-31 and NCCTG-N9831: Disease-free and overall survival data Combined analysis of NSABP-B-31 and NCCTG-N9831: Disease-free and overall survival data
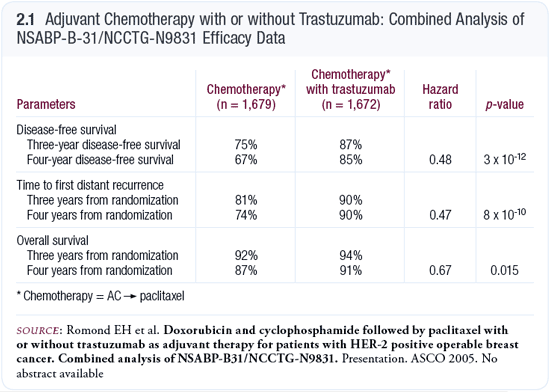
In the combined analysis of the NSABP-B-31 and NCCTG-N9831 adjuvant trastuzumab trials, disease-free survival was the primary endpoint, but we also examined distant disease-free survival because it’s a good surrogate for overall survival. What’s impressive about the data is that the absolute difference in disease-free survival is 12 percent at three years, favoring trastuzumab, and those data are quite firm because many women are now three years out (Romond 2005; [2.1]). The estimate at four years is a striking 18 percent.
In addition, even though the median follow-up in the combined data set was only two years, a statistically significant difference in survival was already evident. That partly reflects the adverse prognosis of this disease — if the patient relapses, it occurs earlier rather than 10 years later. In the control arm, the recurrence rate was 25 percent at three years, which demonstrates the aggressiveness of this disease.
We never expected to see a survival benefit so early in this trial, yet at three years, the difference in overall survival was statistically significant with 3,351 women.
Combined analysis of NSABP-B-31 and NCCTG-N9831: Distant disease-free survival data
The distant disease-free survival data are also compelling. At three years of follow-up, distant disease-free survival in the trastuzumab arm is 90 percent versus 81 percent in the control arm. At four years, it drops to 74 percent in the control arm, whereas in the trastuzumab arm, it stays at 90 percent (Romond 2005; [2.1]). This indicates that we are not yet seeing late recurrences in the trastuzumab-treated patients. Currently, only a few hundred women are four years out, so those data have more wiggle room than the three-year data. However, if this continues for another year, we may be seeing a plateau in the breast cancer survival curve for the first time.
In both the control and trastuzumab-treated arms, the highest rate of recurrences and distant recurrences occurred in the second year. It was 90 per 1,000 women per year versus approximately 40 per 1,000 women per year in the control and trastuzumab arms, respectively. However, in the control arm, the rate of distant recurrence was essentially the same in the third and fourth years, whereas the rate plummeted in the third year and went down even further in the fourth year in the trastuzumab-treated arm.
If the data hold over time, it will completely change the ballgame in HER2- positive breast cancer. It may mean these patients are being cured early. We can’t say that with confidence yet, but if the data hold up, it could be exciting.
Sequential versus concurrent trastuzumab with chemotherapy: Cardiac toxicity
The Intergroup trial NCCTG-N9831 did not just replicate NSABP-B-31; it was also designed to examine whether trastuzumab is better given concurrently or sequentially with chemotherapy and to evaluate the risk of cardiac events in each schedule. Patients were randomly assigned to one of three arms: chemotherapy without trastuzumab, trastuzumab given with paclitaxel and then continued for a total of one year or trastuzumab given after paclitaxel for one year. Perez presented data at ASCO in 2005 that showed cardiac events occurred in both schedules of trastuzumab, but they occurred more often in patients who received concurrent rather than sequential trastuzumab (Perez 2005).
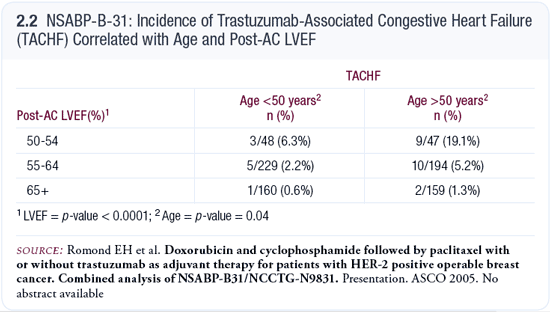
In the NSABP trial B-31, 30 patients treated with adjuvant trastuzumab experienced NYHA Class III and IV CHF, and this correlated with age and even more so with the patient’s post-AC ejection fraction measurement (Romond 2005; [2.2]). If the ejection fraction was over 65 percent, it was unusual for them to experience clinical CHF.
This measurement was highly statistically significant, and it may be a clinically useful parameter when deciding whether to administer trastuzumab with paclitaxel or to give the cardiac muscle a break by finishing paclitaxel and then giving trastuzumab in patients who have already received AC.
Adjuvant trastuzumab in patients with node-negative disease
The NSABP-B-31 and NCCTG-N9831 adjuvant trastuzumab trials were initially limited to patients with node-positive disease. However, in May 2003, the Intergroup amended their protocol to include patients with high-risk, node-negative disease, which were basically ER-negative/HER2-positive or ER-positive/HER2-positive tumors that were larger than two centimeters. As a result, in the overall data set, approximately 100 patients in each arm had node-negative disease.
The relative risk reduction in the combined data analysis of patients with node-negative disease was approximately 0.48 — the same as the entire data set. The problem is that with 100 or less patients with node-negative disease in the arms of the N9831 protocol, the confidence interval goes out forever and crosses one. That does not mean there is no biologic effect; it probably exists, but it’s difficult to pin down how much benefit we gain by using trastuzumab in patients with node-negative disease. The HERA trial may be a better data set to examine the benefit of adjuvant trastuzumab in that population, because one third of those patients had node-negative disease (2.3).
Importance of reliable HER2 testing
The NSABP-B-31 and NCCTG-N9831 trials were designed to require confirmatory HER2 testing in approximately the first 100 patients. However, we found that over 20 percent of the IHC tests reported as 3+ by community hospitals were not HER2-positive when evaluated centrally by FISH or repeat IHC. We found that when the IHC was performed in laboratories with a lot of experience, such as reference laboratories that do 100 or more assays a month, the results correlated with FISH positivity in over 95 percent of cases. Therefore, we put a constraint in the protocol that IHC assays performed at community hospitals had to be confirmed at a good reference laboratory.
From an economic and toxicity standpoint, it’s extremely important that HER2-positive results are really HER2-positive and the target is there. IHC 2+ results should have a FISH assay performed, and a few patients with IHC 1+ results will have gene amplification by FISH also. Off protocol, I would use adjuvant trastuzumab in patients with positive nodes who have FISH-confirmed, HER2- positive disease. Another alternative is to perform a FISH assay, although that’s not 100 percent reliable in the community either (Perez 2004).

Select publications
 |
Dr Romond is an Associate Professor of Medicine in the Division of Hematology/Oncology at the University of Kentucky in Lexington, Kentucky. |
|

