|

What is the optimal local and systemic therapy
for DCIS? Do all DCIS patients need radiation therapy? What is the
optimal endocrine therapy for DCIS?
 |
|
OVERVIEW:
The widespread use of screening mammography has resulted
in an increasing fraction of breast cancer patients
presenting with early-stage disease, including ductal
carcinoma in situ. One of the most controversial current
management issues for DCIS involves the role of lumpectomy
without radiation therapy. The criteria for this approach
is not well-defined. Since DCIS patients are at increased
risk for a second breast cancer, current clinical trials
are addressing the role of endocrine intervention. A
particularly salient issue — in view of the recently
reported ATAC trial results — is whether aromatase
inhibitors can replace tamoxifen in postmenopausal women.
|
|
| About how many women with DCIS
did you treat in the last year? |
|
|
About how many of these women did you:
|
Treat with lumpectomy/radiation therapy?
|
12.55
|
|
Treat with lumpectomy/no radiation
therapy?
|
2.80
|
|
Refer to a medical oncologist?
|
17.85
|
|
Start on tamoxifen?
|
10.60
|
|
|
What do you believe the results would
be of a randomized clinical trial comparing tamoxifen to anastrozole
in women with DCIS?
|
Regarding efficacy:
|
|
|
Greater benefits with anastrozole
|
60%
|
|
No significant difference
|
30%
|
|
Treat with mastectomy?
|
10%
|
| Regarding
toxicity: |
|
|
Less toxicity with anastrozole
|
55%
|
|
No significant difference
|
40%
|
|
Undetermined
|
5%
|
|
|
What type of research data would you
require in order to use anastrozole or another aromatase inhibitor
in a postmenopausal woman with DCIS?
|
Would use it now based on ATAC data
|
55%
|
|
Would only use it if ATAC trial continued
to show
similar results with longer follow-up
|
30%
|
|
Would only use it if a trial demonstrated
safety and efficacy in DCIS patients
|
10%
|
|
Would use it if oncologists started
prescribing
anastrozole over tamoxifen
|
5%
|
|
NSABP PROPOSED TRIAL COMPARING ANASTROZOLE TO
TAMOXIFEN IN DCIS
The driving force of current research is to move away from the
concept that DCIS is simply a surgical problem — and that if
you obtain 10 mm margins, the patient is cured and no adjuvant therapy
is needed. It’s not really important to argue about whether
there’s a set of patients who don’t need radiation therapy.
Even if we take out the index DCIS, the risk for these women to
have another tumor in either breast in the future is at least as
high or higher than the risk for women in the NSABP P-1 prevention
trial. Chemoprevention in DCIS is an important issue, and we need
to find out how to do this best.
Nobody believed when we started with tamoxifen that it would be
a "home run" and end all inquiry. As enormously successful
as the Prevention Trial was in reducing the incidence of cancer
by 50%, everybody understands that there must be a more effective
or safer drug.
The ATAC trial is answering the question about anastrozole in
invasive breast cancer. We need to ask the same question in non-invasive
disease. We are focusing on anastrozole, because there are the two
studies in advanced disease that show that it’s equal or superior
to tamoxifen.
—Richard Margolese, MD
TREATMENT OF DCIS PATIENTS WITH POSITIVE SENTINEL
NODES
This is an extremely challenging issue. There clearly are patients
with DCIS diagnosed by core biopsy techniques who have invasive
cancer in their final specimens. Once they have invasive cancer,
I don’t consider them in the DCIS pool. So the first step when
a "DCIS patient" has a positive node by H & E is to
go back and ask the pathologist to make extra sections and look
very hard for invasive carcinoma. Moffitt has looked retrospectively
at DCIS patients with IHC-positive nodes and found no surv i val
impact, but that study didn’t have much statistical powe r.
We have no reliable data to guide us in that situation, which is
the reason I don’t do IHC in routine practice.
—Monica Morrow, MD
NATURAL HISTORY
In premenopausal women, DCIS is over-diagnosed. It is likely that
only one in five, if undetected, would prog ress to become inva
s i ve b reast cancer. But having detected it, we then have to treat
it. One of the most dishonest things about promoting mammographic
screening for women under the age of 50 is the idea of, "Come
for screening. We’ll save your life and save your breast."
Because DCIS is often outside one quadrant, the breast is not saved.
So you have this extraordinary paradox that women think DCIS is
early breast cancer. Yet, in the U.K. and the U.S.A., about 40%
of premenopausal women with DCIS end up having a mastectomy. W h
e reas, if it was left to appear as an inva s i ve breast cancer,
the treatment would be a lumpectomy. No one can explain that mismatch.
—Michael Baum, ChM, FRCS
SELECTION OF DCIS PATIENTS FOR RADIATION THERAPY
I have a reputation for not wanting to give radiation to any DCIS
patient, but that’s not true. We recommend it to many, but
not all, patients. It’s relatively expensive and it’s
a bit inconvenient. Patients have to come five days a week for at
least five weeks. In some patients, we see significant radiation
fibrosis, although much less in the last five or six years.
Also, if you give radiation therapy for DCIS and you get an invasive
recurrence, radiation can’t be given again. If you don’t
give radiation and there is an invasive recurrence, you can excise
and irradiate. Another consideration is that if you end up having
to do a skin-sparing mastectomy for an invasive recurrence, it’s
a lot more difficult after radiation because of secondary vascular
changes.
— Melvin Silverstein, MD
NSABP TRIALS B-17 AND B-24: RADIATION THERAPY
AND TAMOXIFEN FOR DCIS
Our randomized trials demonstrate that, no matter what the margin
difference or histologic subtype, there is a clear benefit from
the use of radiation therapy. There is also a clear-cut benefit
from tamoxifen for both tumor recurrence and reduction in risk for
contralateral breast cancers. DCIS patients are at high-risk for
contralateral breast cancers, and tamoxifen reduces that risk by
more than 50%. The quest to identify patients who can avoid radiation
therapy is very reasonable. The problem is that even an excellent
observational series is potentially fraught with methodologic bias
that can produce flawed results or conclusions. This isn’t
a surgeon’s disease. It is a woman’s disease. And if you
have a woman in front of you who has the information available today,
I feel that offering radiation therapy increases her chance of being
in that zero group.
—Lawrence Wickerham, MD
 |
NSABP B-17: PHASE III RANDOMIZED STUDY
OF POSTOPERATIVE RADIOTHERAPY FOLLOWING SEGMENTAL MASTECTOMY
AND AXILLARY DISSECTION IN PATIENTS WITH
NONINVASIVE INTRADUCTAL ADENOCARCINOMA OF THE BREAST CLOSED
PROTOCOL |
|
|
|
| |
Fisher B et al. Lumpectomy compared with lumpectomy and
radiation therapy for the treatment of intraductal breast cancer.
N Engl J Med 1993; 328:1581-1586. Abstract
|
 |
| |
 |
| NSABP B-24: PHASE III RANDOMIZED TRIAL
OF ADJUVANT TAMOXIFEN VS PLACEBO FOLLOWING BREAST IRRADIATION
IN PATIENTS WHO HAVE UNDERGONE LUMPECTOMY FOR NONINVASIVE
INTRADUCTAL CARCINOMA (DCIS) OF THE BREAST CLOSED
PROTOCOL |
|
|
|
| |
| Fisher B et al. Tamoxifen in treatment of intraductal breast
cancer: National Surgical Adjuvant Breast and Bowel Project
B-24 randomised controlled trial. Lancet 1999;353(9169):1993-2000.
Abstract |
 |
|
|
 |
| |
|
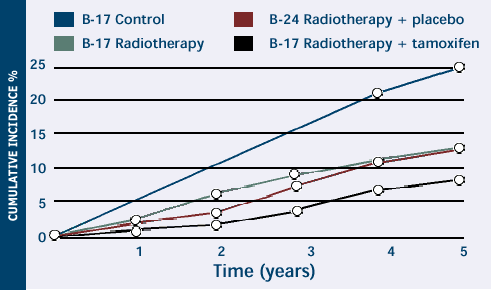
NSABP trials of patients receiving lumpectomy
for DCIS. Cumulative incidence of all invasive and noninvasive
events in the ipsilateral and contralateral breast in NSABP
B-17 and B-24 studies. Note the stepwise improvements in outcome
with the addition of XRT and tamoxifen to lumpectomy for DCIS.
Modified from Lancet 1999;353(9169):1993-2000. Abstract
|
| |
 |
| PROPOSED IBIS 2 TRIAL: INTERNATIONAL BREAST
INTERVENTION STUDY 2 |
|
|
|
| |
| |
 |
|
|
 |
| RTOG-9804; RTOG-DEV-1026: PHASE III RANDOMIZED
STUDY OF TAMOXIFEN WITH OR WITHOUT RADIOTHERAPY IN WOMEN
WITH DUCTAL CARCINOMA IN SITU (DCIS) OF THE BREAST OPEN
PROTOCOL |
|
|
|
| |
 |
|
STUDY CONTACT
Barbara L. Smith, Chair, Phone: 617-724-4800
Beryl McCormick, Chair, Phone: 212-639-6828
Radiation Therapy Oncology Group
|
| |
 |
| PROPOSED NSABP DCIS TRIAL: TAMOXIFEN VERSUS
ARIMIDEX IN POSTMENOPAUSAL PATIENTS WITH DUCTAL CARCINOMA
IN SITU |
|
|
|
| |
| Margolese R. Rationale for proposed National Surgical Adjuvant
Breast and Bowel Project (NSABP): DCIS Trial. Tamoxifen versus
Arimidex® (anastrozole) in postmenopausal patients with
ductal carcinoma in situ. Poster, 2001 Miami Breast Cancer
Conference. Full-Text |
 |
|
|
View References
Back | Top of Page

|
|



