|
| |
|
| Breast Cancer Clinical Trials |
 |
| Neoadjuvant systemic therapy |
|
| |
|
 |
| PHASE III RANDOMIZED STUDY OF DOXORUBICIN
AND CYCLOPHOSPHAMIDE FOLLOWED BY PACLITAXEL OR DOCETAXEL
IN WOMEN WITH NODE-POSITIVE OR HIGH-RISK NODE-NEGATIVE
STAGE II OR IIIA BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
E-1199, SWOG-E1199, CLB-49906, NCCTG-E1199 |
| PROJECTED ACCRUAL: |
A total of 5,000 patients will be accrued within 1.27
years. |
|
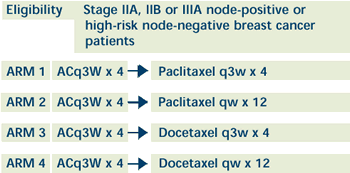
|
ER- and/or PR-positive patients receive tamoxifen
x 5
years after completion of chemotherapy |
 |
|
OBJECTIVES:
- Compare the disease-free survival and overall survival
in patients with node-positive or high-risk node-negative
operable stage II or IIIA breast cancer treated with docetaxel
or paclitaxel after AC.
- Determine whether the weekly administration of paclitaxel
or docetaxel for 12 weeks improves disease-free survival
and overall survival when compared with the conventional
schedule of every 3 weeks for 4 courses after AC.
- Compare the toxic effects of docetaxel and paclitaxel
when administered weekly for 12 weeks versus every 3 weeks
for 4 courses in these patients.
- Compare the toxicity of paclitaxel administered every
3 weeks for 4 courses or weekly for 12 weeks to that of
docetaxel administered on the same schedules in these patients.
PARTICIPATION CRITERIA:
- 18 and over, any hormone receptor status
- Histologically confirmed operable stage IIA, IIB or IIIA
adenocarcinoma of the breast with histologically involved
lymph nodes OR high-risk node-negative disease
- Tumor at least 2.1 cm in diameter for node-negative disease
- Bilateral breast disease allowed if at least 1 primary
tumor meets the criteria above
- Must have had at least 6 axillary lymph nodes removed
at dissection and at least one node positive OR sentinel
node biopsy negative for metastasis (SNLB+ allowed if enrolled
on American College of Surgery Trial Z0011 and have been
randomized to receive no axillary dissection)
- Tumor-free margins at least 1 mm for both invasive and
noninvasive carcinoma except for LCIS (< 1 mm allowed)
• Concurrent enrollment on ACS Z0010, Z0011 or NSABP
B-32 allowed
STUDY CONTACTS
Joseph A Sparano, Chair, Ph: 718-904-2555
Eastern Cooperative Oncology Group
Edith A Perez, Chair, Ph: 507-284-5369
North Central Cancer Treatment Group
Silvana Martino, Chair, Ph: 310-998-3961
Southwest Oncology Group
Vicky Eileen Jones, Chair, Ph: 858-657-8710
Cancer and Leukemia Group B
|
 |
| PHASE III RANDOMIZED STUDY OF NEOADJUVANT
DOXORUBICIN AND YCLOPHOSPHAMIDE WITH OR WITHOUT FILGRASTIM
(G-CSF) IN WOMEN WITH INFLAMMATORY OR ESTROGEN RECEPTOR-NEGATIVE
LOCALLY ADVANCED BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
SWOG-S0012, CTSU |
| PROJECTED ACCRUAL: |
A total of 300 patients (150 per arm) will be accrued
for this study. |
|

|
|
A=doxorubicin; C=IV cyclophosphamide; Co=oral
cyclophosphamide; G-CSF=filgrastim
Within 3-6 weeks after completion of chemotherapy, patients
with stable or responsive disease undergo surgical resection
of tumor and affected nodes.
|
 |
|
OBJECTIVES:
- Compare the response rates in women with inflammatory
or estrogen receptor-negative, locally advanced breast cancer
treated with neoadjuvant doxorubicin and
cyclophosphamide with vs without filgrastim (G-CSF).
- Compare the toxic effects of these regimens.
- Compare the delivered dose intensity of these regimens.
- Evaluate the association between microscopic pathologic
complete response and clinical complete response at the
primary tumor site in these patients.
PARTICIPATION CRITERIA:
- Age and menopausal status not specified
- Histologically confirmed inflammatory or locally advanced
breast cancer
• Stage IIB (T3, N0, M0), IIIA (T3, N1-2, M0 or T0-2,
N2, M0) or IIIB (T4, any N, M0 or any T, N3, M0)
- Un resectable or otherwise appropriate for neoadjuvant
therapy
- Confirmed by core needle or incisional biopsy
- Estrogen receptor-negative if disease is not inflammatory
STUDY CONTACT
Georgiana Kehr Ellis, Chair, Ph: 206-288-2048
Southwest Oncology Group
|
 |
| PHASE III RANDOMIZED STUDY OF NEOADJUVANT
FLUOROURACIL, EPIRUBICIN, AND C YCLOPHOSPHAMIDE VERSUS
NEOADJUVANT DOCETAXEL AND EPIRUBICIN FOLLOWED BY RADIOTHERAPY
AND SURGERY IN WOMEN WITH LOCALLY ADVANCED, NFLAMMATORY,
OR LARGE OPERABLE BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
EORTC-10994 |
| PROJECTED ACCRUAL: |
A total of 1,440 patients will be
accrued for this study within 3 years. |
|
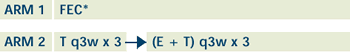
|
|
FEC=fluorouracil, epirubicin, cyclophosphamide;
T=docetaxel
*Patients receive 1 of 3 FEC regimens (FEC 100, Canadian
FEC, Tailored FEC), according to participating organization.
Following chemotherapy, patients may undergo radiotherapy
with or without breast conservation or mastectomy.
|
 |
|
OBJECTIVES:
- Compare neoadjuvant fluorouracil, epirubicin and cyclophosphamide
vs docetaxel and epirubicin followed by radiotherapy and
surgery in women with locally advanced,
inflammatory or large operable breast cancer.
- Compare the progression-free survival with these regimens.
- Compare the distant metastasis-free survival and survival
of patients treated with these regimens.
- Compare clinical and pathological responses to these
regimens.
- Compare the toxicity of these regimens in these patients.
PARTICIPATION CRITERIA:
- Age 70 and under
- Histologically confirmed breast cancer
- Locally advanced or inflammatory disease (T4a-d, any N,
M0; or any T, N2 or N3, M0)
- Large T2 or T3 breast cancer requiring tumor shrinkage
prior to BCT
STUDY CONTACTS
Herve Bonnefoi, Chair, Ph: 011-41-22-382-33-11
EORTC Breast Cancer Group
Jonas Bergh, Chair, Ph: 46-8-51776279
Swedish Breast Cancer Group
Barbara Muster, Chair Ph: 011-41-31-389-9191
Swiss Institute for Applied Cancer Research
|
 |
| PHASE III RANDOMIZED STUDY OF NEOADJUVANT
FLUOROURACIL/ DOXORUBICIN/CYCLOPHOSPHAMIDE (FAC) VS CYCLOPHOSPHAMIDE/
METHOTREXATE/FLUOROURACIL (CMF) IN PATIENTS WITH STAGE
III BREAST CANCER. Open
Protocol |
|
| PROTOCOL IDS: |
GOCS-08-BR-95-III, NCI-F95-0036 |
| PROJECTED ACCRUAL: |
If unacceptable toxicity is observed in 10 or more patients,
the study will be closed. |
|

|
| |
 |
|
OBJECTIVES:
- Compare the response to neoadjuvant therapy with fluorouracil,
doxorubicin and cyclophosphamide (FAC) vs cyclophosphamide,
methotrexate and fluorouracil (CMF) in patients with stage
III breast cancer.
- Compare the rate of breast conservation and local-regional
control with these two regimens.
- Assess the disease-free and overall survival.
- Assess the toxic effects, cosmetic results after conservative
surgery, quality of life and patient compliance.
PARTICIPATION CRITERIA:
- Age 21-75
- Breast cancer histologically confirmed, measurable, stage
III breast cancer
- No inflammatory breast cancer
* In both arms, pts with resectable disease after the
3rd course of chemotherapy undergo quadrantectomy with
AND or MRM, folllowed by 6 additional courses of the chemotherapy
regimine to which they were initially randomized. Patients
without distant metastasis receive locoregional radiotherapy
concurrently with postoperative chemotherapy. Patients
on both arms with unresectable dz after the initial 3
courses of
chemotherapy receive locoregional radiotherapy then surgical
resection (if feasible).
STUDY CONTACT
Bernardo A. Leone, Chair, Ph: 54-299-4436396
Grupo Oncologico Cooperativo del Sur
|
 |
| PHASE II PILOT STUDY OF CDNA MICROARR
AY AS A MEASURE OF TUMOR RESPONSE TO NEOADJUVANT DOCETAXEL
AND CAPECITABINE FOLLOWED BY SURGERY AND ADJUVANT DOXORUBICIN
AND CYCLOPHOSPHAMIDE IN PATIENTS WITH STAGE II OR III
BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
NCI-00-C-0149 |
| PROJECTED ACCRUAL: |
A total of 18-36 patients will be accrued within 18
months. |
|

|
|
T=docetaxel; X=capecitabine;
AC=doxorubicin/cyclophosphamide
Patients who undergo lumpectomy and ALND receive standard
XRT following the completion of chemotherapy. Patients who
undergo a modified radical mastectomy
may receive chest wall radiation. ER+ or PR+ patients receive
tamoxifen po qd x 5 yrs.
Tumor tissue is collected at baseline, day 2 of course 1
of neoadjuvant chemotherapy, prior to course 2 of neoadjuvant
chemotherapy, and at surgery. Samples are subjected to reverse
transcriptase PCR and cDNA microarray analysis.
|
 |
|
OBJECTIVES:
- Evaluate the feasibility of using cDNA microarray as
a measure of a tumor’s biological response to neoadjuvant
docetaxel and capecitabine followed by surgery and adjuvant
doxorubicin and cyclophosphamide by characterizing the cDNA
expression patterns before and after chemotherapy in patients
with stage II or III breast cancer.
- Determine the toxicities of this regimen in these patients.
- Determine the clinical and pathologic response rate.
PARTICIPATION CRITERIA:
- Hormone receptor status known
- Histologically or cytologically confirmed stage II or
III breast cancer (Tumor size greater than 2 cm)
- Prior biopsy allowed if adequate tissue remains for 2nd
biopsy
STUDY CONTACT
JoAnne Zujewski, Chair, Ph: 301-402-0985
Center for Cancer Research Medicine Branch
Bethesda, Maryland
|
 |
| PHASE III RANDOMIZED STUDY OF BREAST-CONSERVING
LO CA L T H E RAPY VERSUS MASTECTOMY FOLLOWED BY RA D
I O T H E RAPY IN WOMEN WITH LO CALLY ADVANCED BREAST
CANCER WHO H AVE RECEIVED PRIOR INDUCTION CHEMOTHERAPY
Open
Protocol |
|
| PROTOCOL IDS: |
EORTC-10974, EORTC-BIG-0002, LAMANOMA |
| PROJECTED ACCRUAL: |
A total of 1,300 patients (650 per treat-ment arm) will
be accrued for this study within 5 years. |
|

|
| PR= Partial Response; CR= Complete Response
|
 |
|
OBJECTIVES:
- Compare the overall survival and time to loco-regional
failure in women with locally advanced breast cancer treated
with breast-conserving local therapy vs mastectomy followed
by radiotherapy after they have received prior induction
chemotherapy.
- Compare the quality of life of patients treated with
these regimens.
PARTICIPATION CRITERIA:
- Locally advanced breast cancer
- T3 inoperable, N0-N2
- Any T, N2
- T4, N0-N2
- Inflammatory breast carcinoma
- Prior treatment, 4-6 courses standard induction chemotherapy
/ active investigational regimens completed within the past
4 weeks
- Residual tumor size less than 5 cm
- No fixed axillary lymph nodes
- No multifocal or bilateral breast cancer
- No clinical suspicion of extensive ductal carcinoma in
situ
- No unresolved skin edema
- No distant metastases
- Positive bone scan allowed provided there are no bone
metas-tases on x-ray
STUDY CONTACT
England
Marie Emson, Ph: +44 (0) 2088461478
Charing Cross Hospital
London, England, United Kingdom
|
 |
| PHASE III RANDOMIZED STUDY OF DOXORUBICIN
AND CYCLOPHOSPHAMIDE WITH OR ITHOUT DEXRAZOXANE, FOLLOWED
BY PACLITAXEL WITH OR WITHOUT TRASTUZUMAB (HERCEPTIN),
FOLLOWED BY SURGERY AND RADIOTHERAPY WITH OR WITHOUT TRASTUZUMAB
IN WOMEN WITH HER-2+ STAGE IIIA OR IIIB OR REGIONAL STAGE
IV BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
CLB-49808, CTSU |
| PROJECTED ACCRUAL: |
A total of 396 patients will be accrued within 4 years. |
|
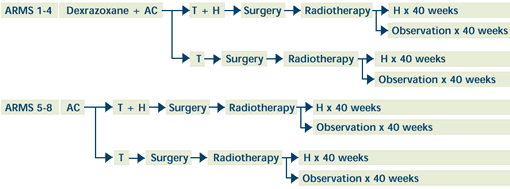
|
|
AC=doxorubicin/cyclophosphamide; T=paclitaxel; H=trastuzumab
Within 12 weeks of completion of neoadjuvant chemotherapy,
ER/PR-positive patients may receive tamoxifen qd x 5 years.
Treatment continues in all arms in the absence of distant
disease progression.
|
 |
|
OBJECTIVES:
- Determine the time to locoregional recurrence, time to
completion of treatment, and overall survival in women with
HER2+ stage IIIA or IIIB or regional stage IV breast cancer
treated with doxorubicin and cyclophosphamide (AC) with
or without dexrazoxane, followed by paclitaxel with or without
trastuzumab (Herceptin), followed by surgery and radiotherapy
with or without trastuzumab.
- Determine whether addition of trastuzumab to paclitaxel
therapy improves response at 24 weeks of therapy.
- Determine whether addition of trastuzumab to paclitaxel
therapy increases the rate of cardiotoxicity.
- Determine whether addition of dexrazoxane to AC compromises
response.
- Determine whether addition of dexrazoxane to AC reduces
the rate of cardiotoxicity.
- Determine whether long-term trastuzumab after local therapy
improves disease-free survival.
- Determine whether long-term trastuzumab after local therapy
increases the rate of cardiotoxicity.
- Determine the occurrence of any grade 3 or higher toxicity,
second malignancies, acute myelogenous leukemia, or myelodysplastic
syndrome.
- Determine the eventual rate of breast conservation in
those patients considered candidates for breast conservation
prior to neoadjuvant therapy.
- Determine the clinical response after AC with or without
dexrazoxane and the clinical/mammographic/ultrasound response
after paclitaxel with or without rastuzumab, compared to
the pathologic response at definitive surgery.
PARTICIPATION CRITERIA:
- 18 years and older
- Histologically confirmed primary infiltrating adenocarcinoma
of the breast confirmed by core needle biopsy or incisional
biopsy
- Amplification of HER2 by FISH OR IHC 3+
- Staging criteria after complete clinical and radiographic
staging:
- T3, N1, M0 OR
- Any T, N2 or N3, M0 OR
- T4, any N, M0 including clinical or pathological inflammatory
disease OR
- regional stage IV disease with supraclavicular or infraclavicular
lymph nodes as only site of metastasis
- Measurable or evaluable disease
- Prior DCIS of the ipsilateral breast allowed if treated
with excision only
- Metaplastic carcinoma allowed
- Synchronous bilateral primary disease allowed (provided
at least one cancer meets staging criteria)
- No dermal lymphatic involvement with clinical inflammatory
changes
STUDY CONTACT
Mark L. Graham, Chair, Ph: 919-859-6631
Cancer and Leukemia Group B
|
PHASE II RANDOMIZED STUDY OF VINORELBINE/EPIRUBICIN
VERSUS INORELBINE/MITOXANTRONE VERSUS CYCLOPHOSPHAMIDE/DOXORUBICIN
AS PREOPERATIVE CHEMOTHERAPY IN WOMEN WITH EARLY STAGE BREAST CANCER
Open
Protocol
PROTOCOL IDS: RMNHS-TOPIC2; EU-99037
STUDY CONTACT:
Ian Edward Smith, Ph: 0208-661-3280
Royal Marsden Hospital
Sutton, England, United Kingdom

PHASE II STUDY OF NEOADJUVANT DOXORUBICIN, CYCLOPHOSPHAMIDE,
AND PACLITAXEL WITH OR WITHOUT TRASTUZUMAB (HERCEPTIN) FOLLOWED
BY LOCAL SURGERY WITH OR WITHOUT ADJUVANT TRASTUZUMAB OR ADJUVANT
DOXORUBICIN, CYCLOPHOSPHAMIDE, PACLITAXEL, AND TRASTUZUMAB IN WOMEN
WITH STAGE IIB, IIIA, IIIB, OR IV BREAST CANCER Open
Protocol
PROTOCOL IDS: UNC-9818; NCI-G00-1836
STUDY CONTACT:
Mark L. Graham, Ph: 919-859-6631
Lineberger Comprehensive Cancer Center, UNC
Chapel Hill, North Carolina

PHASE II STUDY OF NEOADJUVANT SEQUENTIAL DOXORUBICIN
AND DOCETAXEL IN WOMEN WITH STAGE III BREAST CANCER Open
Protocol
PROTOCOL IDS: MCC-11971; NCI-G00-1763;
MCC-IRB-5292
STUDY CONTACT:
Susan Minton, Ph: 813-972-4673
H. Lee Moffitt Cancer Center and Research Institute
Tampa, Florida

PHASE II RANDOMIZED STUDY OF DOCETAXEL WITH OR WITHOUT
BEVACIZUMAB, FOLLOWED BY SURGERY, RADIOTHERAPY, AND DOXORUBICIN
AND CYCLOPHOSPHAMIDE IN PATIENTS WITH LOCALLY ADVANCED BREAST CANCER
Open
Protocol
PROTOCOL IDS: CWRU-3100, NCI-2722
STUDY CONTACT:
Beth A. Overmoyer, Chair Ph: 216-844-3862
Ireland Cancer Center

PHASE II RANDOMIZED STUDY OF DOXORUBICIN, CYCLOPHOSPHAMIDE,
AND PACLITAXEL (ACT) VS CYCLOPHOSPHAMIDE, THIOTEPA, AND CARBOPLATIN
(STAMP V) IN PATIENTS WITH HIGH-RISK PRIMARY BREAST CANCER Open
Protocol
PROTOCOL IDS: CHNMC-IRB-98096; NCI-H99-0038;
CHNMC-PHII-18
STUDY CONTACT:
George Somlo, Ph: 626-359-8111
Beckman Research Institute, City of Hope
Los Angeles, California

PHASE III RANDOMIZED NEOADJUVANT STUDY OF ICI 182780
IN WOMEN WITH STAGE I OR II PRIMARY BREAST CANCER Open
Protocol
PROTOCOL ID: EORTC-10963
STUDY CONTACTS:
Cornelis J H van de Velde, Ph: 31-71-5262309
EORTC Breast Cancer Group
Leiden University Medical Center
Leiden, Netherlands
Anthony Howell, Ph: 446-3746 ext 0161
Breast International Group
Christie Hospital, NHS Trust
Manchester, England, United Kingdom
Back | Top of Page

|
|



