|
| |
|
| Breast Cancer Clinical Trials |
 |
| Prevention, DCIS |
|
| |
|
 |
| STUDY OF TAMOXIFEN AND RALOXIFENE (STAR)
FOR THE PREVENTION OF BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
NSABP P-2 |
| PROJECTED ACCRUAL: |
Approximately 22,000 patients will be accrued to this
trial. |
|

|
| Quality of life assessed at baseline and six-month
intervals to five years then annually thereafter. |
 |
|
OBJECTIVES:
- Determine whether raloxifene is more or less effective
than tamoxifen in significantly reducing the incidence rate
of invasive breast cancer in postmenopausal women.
- Evaluate the effects of tamoxifen and raloxifene on the
incidence of intraductal carcinoma in situ, lobular carcinoma
in situ, endometrial cancer, ischemic heart disease, fractures
of the hip and spine, or Colles' fractures of the wrist.
- Evaluate the toxic effects of these regimens in these
participants.
- Determine the effect of these regimens on the quality
of life of these participants.
PARTICIPATION CRITERIA:
- Postmenopausal women at increased risk for developing
invasive breast cancer
- Histologically confirmed lobular carcinoma in situ treated
by local excision only OR at least 1.66% probability of
invasive breast cancer within 5 years using Breast Cancer
Risk Assessment Profile
- No clinical evidence of malignancy on physical exam within
180 days
- No evidence of suspicious or malignant disease on bilateral
mammogram within the past year
- No bilateral or unilateral prophylactic mastectomy
- No prior invasive breast cancer or intraductal carcinoma
in situ
STUDY CONTACT
Norman Wolmark, Chair, Ph: 412-359-3336
National Surgical Adjuvant Breast and Bowel Project
Allegheny General Hospital, Pennsylvania
|
 |
| PHASE III RANDOMIZED STUDY OF TAMOXIFEN
WITH OR WITHOUT RADIOTHERAPY IN WOMEN WITH DUCTAL CARCINOMA
IN SITU (DCIS) OF THE BREAST Open
Protocol |
|
| PROTOCOL IDS: |
RTOG-9804, RTOG-DEV-1026, CTSU |
| PROJECTED ACCRUAL: |
A total of 1,990 patients will be accrued for this
study over 6 years. |
|
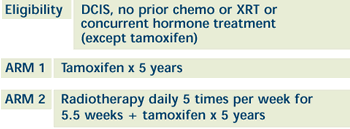
|
| |
 |
|
OBJECTIVES:
- Compare the efficacy of tamoxifen with or without whole
breast radiation in decreasing or delaying the appearance
of local failure, both invasive and in situ, and preventing
the need for mastectomy in women with ductal carcinoma in
situ (DCIS) of the breast.
- Compare distant disease-free survival of these patients
in these treatment arms.
PARTICIPATION CRITERIA:
- Age: 26 and over
- Ductal carcinoma in situ of the breast detected by mammogram
at the time of diagnosis
- Unicentric
- Lesions no greater than 2.5 cm
- Low or intermediate grade
- Inked margins at least 3 mm
- Clinically node-negative
STUDY CONTACTS
Beryl McCormick, Chair, Ph: 212-639-6828
Radiation Therapy Oncology Group
Barbara L Smith, Chair, Ph: 617-724-4800
Cancer and Leukemia Group B
|
 |
| PILOT SCREENING STUDY OF BREAST IMAGING
OUTCOME MEASURES IN WOMEN AT HIGH GENETIC RISK OF BREAST
CANCER Open
Protocol |
|
| PROTOCOL IDS: |
NCI-01-C-0009 |
| PROJECTED ACCRUAL: |
Approximately 200 participants (100 BRCA1/2 mutation
carriers and 100 BRCA1/2 mutation non-carriers) will be
accrued for this study. |
|
| |
| |
 |
|
OBJECTIVES:
- Determine whether breast imaging outcome measures in
women who are BRCA1 or BRCA2 mutation carriers and non-carriers
can be used to define a high-risk imaging
phenotype.
- Assess the use of positron emission tomography imaging
of breast lesions detected by mammography or magnetic resonance
imaging, normal contralateral breast tissue, and normal
ovarian tissue in BRCA1/2 carriers and non-carriers.
- Assess the use of breast duct lavage to obtain epithelial
cell samples for cytologic evaluation and molecular/genetic
studies in high-risk premenopausal women.
- Compare imaging findings with histologic or cytologic
findings from these participants.
PARTICIPATION CRITERIA:
- Age Range: 18 to 49
- Previous participation on protocol NCI-78-C-0039 or NCI-99-C-0081
- Known carrier or at least a 50% probability of carrying
a BRCA1 or BRCA2 mutation
- Must agree to release results of genetic counseling for
stratification purposes
- Have undergone genetic counseling and risk assessment
- At least 6 months since prior steroids, selective estrogen
receptor modulators, or hormonal agents, including the following:
tamoxifen, raloxifene, estrogen, DHEA, anabolic steroids,
oral contraceptives, Depoprovera, progestin IUD, oral progestin,
Norplant, or drugs to induce ovulation
PROTOCOL
Participants undergo a physical exam including exam of breast
and pelvis, standard four-view mammogram, breast magnetic
resonance imaging (MRI), CA-125 l e vel determination and
t r a n s vaginal color doppler ultrasonography.
Participants with abnormal mammogram and/or MRI results are
asked to undergo positron emission tomography scans of the
breast and asymptomatic ovaries.
Breast duct lavage fluid is collected from all participants
for cytologic analysis. Participants undergo repeat screening
studies annually for 3 years.
STUDY CONTACT
Ruthann M. Giusti, Chair Ph: 301-496-1611
Division of Cancer Epidemiology and Genetics
|
PHASE II RANDOMIZED CHEMOPREVENTION STUDY OF LY353381
HYDROCHLORIDE IN WOMEN WITH FINE NEEDLE ASPIRATION CYTOLOGIC EVIDENCE
OF HYPERPLASIA AND AT HIGH RISK FOR BREAST CANCER Open
Protocol
PROTOCOL IDS: KUMC-HSC-7264-97,
NCI-P00-0146
STUDY CONTACTS:
Kansas: Carol J. Fabian, Ph: 913-588-7791
University of Kansas Medical Center
Kansas City, Kansas, U.S.A.
Texas: Joyce A. O'Shaughnessy, Ph: 214-370-1795
US Oncology
Dallas, Texas, U.S.A.

SCREENING STUDY FOLLOWING LOCAL EXCISION IN SELECTED
PATIENTS WITH DUCTAL CARCINOMA IN SITU (DCIS) OF THE BREAST Open
Protocol
PROTOCOL ID: E-5194
STUDY CONTACT:
Lorie L. Hughes, Chair, Ph: 404-233-4960
Eastern Cooperative Oncology Group

GENETIC MAPPING OF INTERACTIVE SUSCEPTIBILITY LOCI
IN PATIENTS AND SIBLINGS WITH BREAST, COLON, LUNG, OR PROSTATE CANCER
Open
Protocol
PROTOCOL IDS: E-1Y97
STUDY CONTACT:
Theodore G. Krontiris, Ph: 626-359-8111
Beckman Research Institute, City of Hope
Los Angeles, California

PHASE II RANDOMIZED STUDY OF EXEMESTANE AND RALOX-IFENE
IN POSTMENOPAUSAL WOMEN WITH A HISTORY OF STAGE 0 (DUCTAL CARCINOMA
IN SITU), I, II, OR III BREAST CANCER WHO HAVE NO CLINICAL EVIDENCE
OF DISEASE Open
Protocol
PROTOCOL IDS: MSKCC-99017; NCI-G99-1662
STUDY CONTACT:
Clifford Hudis, Ph: 212-639-6483
Memorial Sloan-Kettering Cancer Center
New York, New York

PHASE II STUDY OF RALOXIFENE AS A CHEMOPREVENTIVE
AGENT FOR PREMENOPAUSAL WOMEN AT HIGH RISK FOR DEVELOPING INVASIVE
BREAST CANCER Open
Protocol
PROTOCOL IDS: NCI-98-C-0123; MB-402
STUDY CONTACT:
JoAnne Zujewski, Ph: 301-402-0985
Medicine Branch
Bethesda, Maryland
Back | Top of Page

|
|



