|
| |
|
| Breast Cancer Clinical Trials |
 |
| Adjuvant endocrine therapy |
|
| |
|
 |
| PHASE III RANDOMIZED STUDY OF ADJUVANT
TAMOXIFEN, OVARIAN SUPPRESSION, AND/OR CHEMOTHERAPY IN
WOMEN WITH STAGE I, II AND IIIA BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
UKCCCR-ABC; EU-94029 |
| PROJECTED ACCRUAL: |
Approximately 6,000 women (4,000 premenopausal, 2,000
postmenopausal) will be accrued. |
|
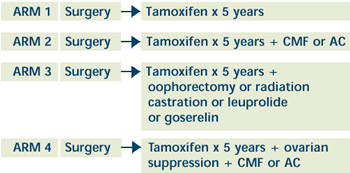
|
- Postmenopausal women randomized to ARM 1 or 2
- Pre- and perimenopausal women randomized based on the
clinician's judgment of appropriate adjuvant therapy as
follows:
- chemotherapy alone (ARM 1 or 2)
- ovarian suppression alone (ARM 1 or 3)
- ovarian suppression with nonrandomized chemotherapy (ARM
2 or 4)
- chemotherapy with nonrandomized ovarian suppression (ARM
2 or 4)
|
 |
|
OBJECTIVES:
- Estimate overall and relapse-free survival of women with
early-stage breast cancer receiving adjuvant tamoxifen with
or without adjuvant chemotherapy and/or ovarian
suppression.
PARTICIPATION CRITERIA
- Pre-, peri- or postmenopausal
- Hormone receptor status: Not specified
- Histologically confirmed invasive breast cancer (stage
I, II or IIIA) for which adjuvant therapy is appropriate
- Pathologically positive or negative nodes
- Any tumor size
STUDY CONTACT
John Robert Yarnold, Chair, Ph: 020-8661-3891
United Kingdom Coordinating Committee on Cancer
Research-ABC
|
 |
| PHASE III RANDOMIZED ADJUVANT STUDY OF
TAMOXIFEN IN WOMEN WITH EARLY BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
CRC-TU-ATTOM, EU-98042 |
| PROJECTED ACCRUAL: |
A total of 8,000-20,000 patients will be accrued into
this study. |
|
 |
| |
 |
|
OBJECTIVES:
- Compare the disease-free and overall survival of women
with early breast cancer who are randomized to stop adjuvant
tamoxifen with those randomized to continue for at least
5 extra years.
PARTICIPATION CRITERIA
- Pre- or postmenopausal
- ER and/or PR-positive, -negative or unknown
- Histologically confirmed breast carcinoma that has been
completely excised
- Clinically relapse-free
- Must have completed at least two years of adjuvant therapy
with tamoxifen for early breast cancer AND no clear indication
for or against receiving further tamoxifen
- No significant endometrial hyperplasia
- No patients with negligibly low risk of breast cancer
death
STUDY CONTACT:
David J Kerr, Ph: 0121-414-3802
Cancer Research Campaign Trials Unit:
University of Birmingham
Birmingham, England, United Kingdom
|
PHASE III RANDOMIZED STUDY OF ADJUVANT LETROZOLE
VERSUS TAMOXIFEN IN POSTMENOPAUSAL WOMEN WITH OPERABLE, HORMONE
RECEPTOR POSITIVE BREAST CANCER Open
Protocol
PROTOCOL IDS: IBCSG-1-98; EU-99022;
IBCSG-18-98; NOVARTIS-2026703019
STUDY CONTACT:
Henning T. Mouridsen, Ph: 35454776
Rigshopitalet
Copenhagen, Denmark
 |
| PHASE III RANDOMIZED STUDY OF MEDROXYPROGESTERONE
ACETATE VERSUS OBSERVATION FOR PREVENTION OF ENDOMETRIAL
PATHOLOGY IN PATIENTS WITH POSTMENOPAUSAL BREAST CANCER
TREATED WITH ADJUVANT TAMOXIFEN Open
Protocol |
|
| PROTOCOL IDS: |
SWOG-S9630; SWOG-9630 |
| PROJECTED ACCRUAL: |
A total of 208 patients (104 per arm) will be accrued
within 3 years. |
|
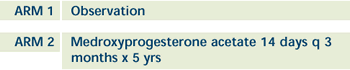
|
| All patients recieve adjuvant tamoxifen x 5
years and undergo an endovaginal sonogram and an endometrial
biopsy (if required) at 2 and 5 years, ARM 2 undergo biopsies
as needed. Patients followed q 6 months for 2 years and then
annually thereafter. |
 |
|
OBJECTIVES:
- Compare endometrial pathologic diagnoses for post menopausal,
tamoxifen- t reated breast carcinoma patients randomly assigned
to observation or cyclical medroxyprogesterone acetate (MA).
- Compare endometrial pathologic diagnoses resulting in
tamoxifen discontinuation and intermittent bleeding in breast
cancer patients receiving tamoxifen and randomized to observation
or cyclical MA.
- Characterize the incidence of spontaneous regression
and progression of simple or cystic hyperplasia.
- Characterize endometrial biopsy results using different
endometrial stripe width cut-off points for cases in which
the width is at least 5 mm by endovaginal ultrasound for
women receiving tamoxifen.
- Compare changes over time in endometrial oncogene expression
and receptor status for postmenopausal tamoxifen-treated
breast carcinoma patients with or without prior chemotherapy
randomly assigned to MA vs observation.
- Describe associations among change in gene expression,
receptor status, endometrial abnormality, length of tamoxifen
exposure and prior chemotherapy.
PARTICIPATION CRITERIA
- Postmenopausal women aged 18 and over
- Candidate for adjuvant tamoxifen (Patients must start
tamoxifen or have started within 28 days prior to study
and plan to continue for 5 years)
- Histologically proven primary invasive stage I, IIA or
IIB breast cancer (T1-3, N0-1, M0)
- Prior definitive local treatment of primary lesion
- Patients with BCT must have received or be planning to
receive XRT at the start of tamoxifen treatment
- No endometrial hyperplasia, proliferative changes, complex
or atypical hyperplasia or carcinoma
STUDY CONTACTS
Ronald Keith Potkul, Chair, Ph: 708-327-3314
Southwest Oncology Group
Leslie Kohman, Chair, Ph: 315-464-6321
Cancer and Leukemia Group B
|
 |
PHASE III RANDOMIZED STUDY OF EXEMESTANE
IN POSTMENOPAUSAL WOMEN WITH RESECTED STAGE I, II OR IIIA
BREAST CANCER WHO HAVE COMPLETED FIVE YEARS OF
TAMOXIFEN Open
Protocol |
|
| PROTOCOL IDS: |
NSABP B-33, CTSU |
| PROJECTED ACCRUAL: |
A total of 3,000 patients will be accrued for this study
within 3 years and 4 months. |
|

|
| Quality of life assessed at baseline and then
q 6 months for 2 years. Patients followed q 6 months for 1 year
and then annually thereafter. |
 |
|
OBJECTIVES:
- Determine whether exemestane following 5 years of tamoxifen
therapy is more effective than 5 years of prior tamoxifen
therapy alone in prolonging disease-free survival, overall
survival and time to treatment failure in postmenopausal
women with resected stage I, II or IIIA breast cancer.
- Determine the effect of tamoxifen withdrawal on bone
in terms of height, fractures, total alkaline phosphatase,
bone mineral density and biochemical markers in these patients.
- Determine the effect of exemestane on bone after tamoxifen
withdrawal in these patients.
- Evaluate the quality of life of a subset of these patients.
PARTICIPATION CRITERIA
- Postmenopausal
- Histologically confirmed invasive stage I-IIIA adenocarcinoma
of the breast (T1-3, N0-1, M0)
- Prior surgical resection
- Currently disease-free
- Primary tumor ER+ and/or PR+
- Borderline ER+ tumors allowed if previously treated with
tamoxifen
- Previously treated with tamoxifen for 57-66 months
- Completed tamoxifen within the past 180 days
- No advanced disease at time of original diagnosis
STUDY CONTACT:
Terry Mamounas, Chair, Ph: 330-363-6281
National Surgical Adjuvant Breast and Bowel Project
|
 |
| PHASE III RANDOMIZED STUDY OF LETROZOLE
VERSUS PLACEBO IN WOMEN WITH PRIMARY BREAST CANCER WHO
HAVE COMPLETED AT LEAST FIVE YEARS OF ADJUVANT TAMOXIFEN
Open
Protocol |
|
| PROTOCOL IDS: |
CAN-NCIC-MA17, EORTC-10983, JRF-Vor-Int-10, NCCTG-CAN-MA17,
SWOG-CAN-MA17, CLB-49805 |
| PROJECTED ACCRUAL: |
Approximately 4,800 patients will be accrued for this
study within 4 years. |
|
 |
| |
 |
|
OBJECTIVES:
- Determine the disease-free survival and overall survival
for women who have previously received at least five years
of adjuvant tamoxifen who are randomized to receive either
letrozole or placebo.
- Evaluate the incidence of contralateral breast cancer
in this patient population.
- Evaluate the longterm clinical and laboratory safety
of letrozole in terms of lipid profile, cardiovascular morbidity
and mortality, incidence of bone features, in bone density
and common toxic effects.
PARTICIPATION CRITERIA
- Postmenopausal
- Hormone receptor status: Positive or unknown (providing
an effort has been made to determine receptor status by
immunocytochemistry)
- Histologically or cytologically confirmed breast carcinoma
resected at time of original diagnosis
- No evidence of metastases
- No localized or distant breast cancer recurrence. Not
registered on protocol NCCTG 89-30-52, any other IBCSG protocol
or any other SWOG adjuvant breast cancer protocol
- Prior adjuvant chemotherapy allowed
- No other concurrent chemotherapy
- Completed at least 4.5 but no more than 6 years of adjuvant
tamoxifen after resection
- No more than 3 months since prior adjuvant tamoxifen
- No concurrent hormone replacement therapy including raloxifene,
idoxifene or megestrol
- No concurrent use of other aromatase inhibitors
- Prior radiation therapy allowed
STUDY CONTACTS
Paul Edward Goss, Ph: 416-946-4534
NCIC-Clinical Trials Group
Princess Margaret Hospital
Toronto, Ontario, Canada
James N. Ingle, Chair Ph: 507-284-8432
North Central Cancer Treatment Group
Monica Castiglione-Gertsch, Chair Ph: 41-31-389-91-91
International Breast Cancer Study Group
Nicholas J. Robert, Chair Ph: 703-280-5390
Eastern Cooperative Oncology Group
Silvana Martino, Chair Ph: 310-998-3961
Southwest Oncology Group
Hyman Bernard Muss, Chair Ph: 802-847-3827
Cancer and Leukemia Group B
Martine J. Piccart-Gebhart, Chair Ph: 32-2-5413206
EORTC Breast Cancer Group
|
 |
| PHASE III STUDY OF PROLONGED ADJUVANT
TAMOXIFEN FOR CURATIVELY TREATED BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
UKCCCR-ATLAS, EU-96064 |
| PROJECTED ACCRUAL: |
Approximately 20,000 patients will be accrued. |
|

|
|
Patients are randomly assigned to continued tamoxifen or
observation.
Patients are followed annually.
|
 |
|
OBJECTIVES:
- Assess the balance of risks and benefits in prolonging
the duration of adjuvant tamoxifen by at least five years
in patients with curatively treated breast cancer who have
already had about five years of adjuvant tamoxifen.
PARTICIPATION CRITERIA
- Curatively treated carcinoma of the breast
- Must be substantial uncertainty as to whether or not to
continue tamoxifen (i.e., no clear indication or definite
contraindication to further treatment with tamoxifen)
- Currently taking adjuvant tamoxifen
STUDY CONTACT:
Christopher J Williams, Chair, Ph: +44-1865-226628
United Kingdom Coordinating Committee on
Cancer Research
Oxford, England, United Kingdom
|
 |
PHASE III RANDOMIZED STUDY OF EXEMESTANE
VERSUS TAMOXIFEN IN POSTMENOPAUSAL WOMEN WITH PRIMARY
BREAST CANCER WHO HAVE ALREADY RECEIVED 2-3 YEARS OF ADJUVANT
TAMOXIFEN AFTER POTENTIALLY CURABLE
SURGERY Open
Protocol |
|
| PROTOCOL IDS: |
ICCG-96OEXE031-C1396-BIG9702, EU-20013, EU-99002, ICCG-BIG-97/02 |
| PROJECTED ACCRUAL: |
Approximately 4,400 patients (2,200 patients in each
arm) will be accrued for this study. |
|
| Following 2-3 years of adjuvant treatment with
tamoxifen, patients are randomized to receive either |

|
| Patients continue treatment for the remainder
of the 5-year period in the absence of disease relapse or unacceptable
toxicity. Quality of life is assessed at some centers. Patients
followed every 3 months for the first year of treatment, every
6 months for the next two years and then annually thereafter
until year 10. |
 |
|
OBJECTIVES:
- Compare, in terms of disease-free and overall survival,
the sequential administration of exemestane with administration
of further tamoxifen until 5 years of therapy is achieved
in postmenopausal women with operable breast cancer who
have already received 2-3 years of adjuvant tamoxifen.
- Compare the regimens in terms of the incidence of contralateral
breast cancer and long-term tolerability of the regimens.
- Determine the tolerability in terms of endometrial status,
bone metabolism, lipid profile and coagulation profile.
- Assess quality of life in these patients treated with
these regimens.
PARTICIPATION CRITERIA
- Postmenopausal
- Histologically confirmed unilateral adenocarcinoma of
the breast that was considered operable
- Estrogen receptor positive or unknown
- Must have had adequate therapy for primary disease
- Must have remained disease-free after therapy for primary
disease
- Must have been receiving tamoxifen for minimum of 2 years
and maximum of 3 years 1 month with no more than
1 month break at any one time
- No evidence of local relapse or distant metastasis at
any time
STUDY CONTACTS:
Raoul C Coombes, Chair, Ph: +44 (0)20 8846 14 18
International Collaborative Cancer Group
Robert Paridaens, Chair, Ph: 32-16-346902
EORTC Breast Cancer Group
Moise Namer, Chair, Ph: 33 4 92031351
Federation Nationale des Centres de Lutte Contre le Cancer
|
Back | Top of Page

|
|



