| |
|
| Breast Cancer Clinical Trials |
 |
| Metastatic disease |
|
| |
|
 |
| PHASE III RANDOMIZED STUDY OF BEVACIZUMAB
WITH CAPECITABINE VERSUS CAPECITABINE ALONE IN WOMEN WITH
PREVIOUSLY TREATED METASTATIC BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
GENENTECH-AVF2119g, GUMC-00299, MSKCC-01008, UAB-0028,
UAB-F001009003 |
| PROJECTED ACCRUAL: |
A total of 400 patients will be accrued for this study
within 12 months. |
|
| |
| |
 |
|
OBJECTIVES:
- Compare the efficacy of bevacizumab with capecitabine
vs capecitabine alone in women with previously treated metastatic
breast cancer, as measured by time to disease progression,
objective response rate, duration of response, 1-year survival,
and duration of survival.
- Compare the safety of these regimens in these patients.
- Compare the pharmacokinetics of these regimens in a subset
of these patients.
- Determine the pharmacodynamics of bevacizumab with capecitabine
in a subset of these patients.
- Compare the extent of change in quality of life of patients
treated with these regimens.
PARTICIPATION CRITERIA
- Age: 18 and over
- Histologically confirmed progressive metastatic breast
cancer
- Previously treated with 1-2 conventional chemotherapy
regimens for metastatic disease
- Previously treated with both an anthracycline (or anthracenedione)
and taxane OR
- No prior chemotherapy for metastatic disease if previously
treated with an adjuvant anthracycline (or anthracenedione)
and taxane regimen and if relapse occurred within 12 months
of completing adjuvant therapy
- Bidimensionally measurable disease
- No HER2-positive disease (3+ by immunohistochemistry or
positive by FISH) unless previously relapsed after trastuzumab
(Herceptin)
PROTOCOL
Arm I: Oral capecitabine twice daily on days 1-14.
Arm II: Chemotherapy as in Arm I plus bevacizumab IV over
30-90 minutes on day 1.
Treatment repeats in both arms every 21 days for up to 35
courses in the absence of disease progression or unacceptable
toxicity. Patients with progressive disease in arm II may
continue to receive bevacizumab alone or in combination with
a new chemotherapy regimen or other treatment.
STUDY CONTACT:
Robert Curry, Chair Ph: 650-225-4922
Genentech Inc.
|
 |
| PHASE II STUDY OF ORAL VINORELBINE IN
ELDERLY WOMEN WITH STAGE IV BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
NCCTG-N003A |
| PROJECTED ACCRUAL: |
A total of 12-35 patients will be accrued for this study
within 7-18 months. |
|
| |
| |
 |
|
OBJECTIVES:
- Determine the objective response rate in elderly women
with stage IV breast cancer treated with oral vinorelbine.
- Determine the toxicity profile of this drug in these
patients.
- Determine the time to progression in these patients treated
with this drug.
- Assess these patients’ preferences with re g a rd
to this oral drug .
- Determine the quality of life of these patients treated
with this drug.
- Assess individual variation in responses (toxicity and/or
activity), pharmacokinetic parameters, and/or biologic correlates
due to genetic differences in enzymes involved in the transport,
metabolism, and/or mechanism of action of this drug in these
patients.
PARTICIPATION CRITERIA
- Age 65 and over
- Histologically or cytologically confirmed stage IV breast
cancer
- Eligible to receive first- or second-line chemotherapy
- Measurable disease: At least 1 lesion that can be accurately
measured in at least 1 dimension as at least 20 mm in longest
diameter. Must be completely outside prior
irradiation port unless there is proof of progressive disease
after completion of prior radiotherapy
PROTOCOL
Patients receive oral vinorelbine weekly. Courses repeat every
4 weeks in the absence of disease progression or unacceptable
toxicity.
STUDY CONTACT:
Edith A. Perez, Chair Ph: 507-284-5369
North Central Cancer Treatment Group
|
 |
| PHASE III RANDOMIZED STUDY OF PACLITAXEL
WITH OR WITHOUT CARBOPLATINAS FIRST-LINE CHEMOTHERAPY
IN ELDERLY WOMEN WITH METASTATIC BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
THERADEX-B00-1370, BMS-TAX/MEN.13 |
| PROJECTED ACCRUAL: |
A total of 220 patients will be accrued for this study. |
|
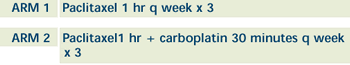
|
| Treatment in both arms continues every 4 weeks
in the absence of disease progression or unacceptable toxicity.
QOL assessed at baseline, before each tx course, and then after
completion. Patients followed q 3 months. |
 |
|
OBJECTIVES:
- Compare the objective response rate in elderly women
treated with paclitaxel with or without carboplatin as first-line
chemotherapy for metastatic breast cancer.
- Compare the overall survival, time to disease progression,
and duration of response in these patients treated with
these regimens.
- Compare the safety of these regimens in this patient
population.
- Compare the quality of life of these patients treated
with these regimens.
PARTICIPATION CRITERIA
- Age: 65 and over
- Stage IV (M1) disease
- HER2-negative (0,1+, or 2+ by IHC or FISH) or status unknown
- Measurable disease (at least 1 lesion >/= 20 mm in
longest diameter or at least 10 mm by spiral CT scan)
- Ineligible if PR or CR to prior hormonal therapy or those
with bone-only disease
- More than 6 months since prior adjuvant chemotherapy or
taxanes
- Prior taxanes allowed only if administered every 3 weeks
STUDY CONTACTS
Edith A. Perez, Chair Ph: 507-284-5369
Theradex
|
 |
| PHASE III RANDOMIZED STUDY OF PACLITAXEL
WITH OR WITHOUT BEVACIZUMAB IN PATIENTS WITH LOCALLY RECURRENT
OR METASTATIC BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
E-2100 |
| PROJECTED ACCRUAL: |
A total of 316-650 patients (158-325 per treatment arm)
will be accrued within 31 months. |
|

|
|
In both arms, treatment repeats q 4 weeks x 18 courses in
absence of disease progression or unacceptable toxicity.
QOL assessed at baseline, day 1 of weeks 17 and 33. Patients
followed q 3 months x 2 yrs, q 6 months x 3 years, and annually
thereafter.
|
 |
|
OBJECTIVES:
- Compare time to treatment failure in patients with locally
recurrent or metastatic breast cancer treated with paclitaxel
with or without bevacizumab.
- Compare the objective response rate, duration of response,
and overall survival in patients treated with these regi-mens.
- Compare the toxicity of these regimens in these patients.
- Compare the quality of life of patients treated with
these regimens.
PARTICIPATION CRITERIA
- Age: 18 and over
- Adenocarcinoma of the breast, locally recurrent disease
not amenable to surgical resection, or Metastatic disease
• No HER2-overexpressing (3+) breast cancer unless
previously treated with trastuzumab
- No CNS metastases
- No prior chemo for locally recurrent or metastatic breast
cancer
- At least 12 months since prior adjuvant or neoadjuvant
taxane therapy
- At least 3 weeks since prior adjuvant chemotherapy
STUDY CONTACT:
Robert L. Comis, Chair Ph: 215-789-3645
Eastern Cooperative Oncology Group
|
PHASE I STUDY OF CD80-MODIFIED ALLOGENEIC BREAST
CANCER CELL LINE TO VACCINATE HLA-A2 POSITIVE WOMEN WITH BREAST
CANCER Open
Protocol
PROTOCOL IDS: PPMC-IRB-94-78; NCI-V98-1379;
OCC-ONC-9408-L
STUDY CONTACT:
Walter John Urba, Ph: 503-215-6259
Earle A. Chiles Research Institute at Providence
Portland, Oregon

PHASE II STUDY OF MITOXANTRONE, LEUCOVORIN CALCIUM,
AND FLUOROURACIL IN ELDERLY WOMEN WITH METASTATIC BREAST CANCER
Open
Protocol
PROTOCOL IDS: FRE-GERCOR-SAM-S99-1,
EU-20028
STUDY CONTACT:
E. Carola, Chair
GERCOR
C. Bouleuc, Ph: 01-34-23-23-65
Centre Hospitalier Victor Dupouy
Argenteuil, France

PHASE II STUDY OF GEMCITABINE IN WOMEN WITH METASTATIC
BREAST CANCER PREVIOUSLY TREATED WITH DOXORUBICIN AND PACLITAXEL
Open
Protocol
PROTOCOL IDS: MSKCC-98030; NCI-G98-1474
STUDY CONTACT:
Violante E. Currie, Chair Ph: 212-639-6991
Memorial Sloan-Kettering Cancer Center
New York, New York

PHASE II STUDY OF IROFULVEN IN PATIENTS WITH METASTATIC
BREAST CANCER Open
Protocol
PROTOCOL IDS: UTHSC-IDD-98-23; NCI-T98-0060;
SACI-IDD-98-23
STUDY CONTACT:
Lisa Hammond, Chair Ph: 210-567-4777
University of Texas Health Science Center at San Antonio
San Antonio, Texas

PHASE II STUDY OF HIGH DOSE COMBINATION CHEMOTHERAPY
AND AUTOLOGOUS OR SYNGENEIC PERIPHERAL BLOOD STEM CELL RESCUE FOLLOWED
BY IMMUNOTHERAPY WITH INTERLEUKIN-2 AND SARGRAMOSTIM (GM-CSF) IN
PATIENTS WITH INFLAMMATORY STAGE IIIB AND RESPONSIVE METASTATIC
STAGE IV BREAST CANCER Open
Protocol
PROTOCOL IDS: FHCRC-1229.00; NCI-G98-1399;
PSOC-1605
STUDY CONTACT:
Frederick R. Appelbaum, Ph: 206-667-4412
Fred Hutchinson Cancer Research Center
Seattle, Washington

PHASE II STUDY OF LY231514 AND GEMCITABINE IN WOMEN
WITH METASTATIC BREAST CANCER Open
Protocol
PROTOCOL ID: NCCTG-983253
STUDY CONTACT:
Alex A. Adjei, Chair, Ph: 507-284-2511
North Central Cancer Treatment Group

PHASE II STUDY OF THE PREDICTIVE VALUE OF THE EXTREME
DRUG RESISTANCE ASSAY IN PATIENTS RECEIVING PACLITAXEL FOR METASTATIC
BREAST CANCER Open
Protocol
PROTOCOL IDS: ONCOTECH-OTBR01; NCI-V98-1391;
UCIRVINE-97-02
STUDY CONTACTS:
Rita S. Mehta, Chair, Ph: 714-798-5933
Oncotech, Inc.
John Butler, Ph: 714-456-8030
Chao Family Comprehensive Cancer Center
Orange, California

PHASE II STUDY OF PS-341 IN WOMEN WITH METASTATIC
BREAST CANCER Open
Protocol
PROTOCOL IDS: NU-NCI00B11, NCI-1862
STUDY CONTACT:
William John Gradishar, Chair Ph: 312-695-4541
Robert H. Lurie Comprehensive Cancer Center, Northwestern
University

PHASE II STUDY OF NONMYELOBLATIVE ALLOGENEIC PERIPHERAL
BLOOD STEM CELL AND DONOR LYMPHOCYTE INFUSIONS IN PATIENTS WITH
REFRACTORY METASTATIC SOLID TUMORS Open
Protocol
PROTOCOL ID: NHLBI-99-H-0064
STUDY CONTACT:
Richard W. Childs, Ph: 800-411-1222
National Heart, Lung, and Blood Insitute
Bethesda, Maryland, U.S.A.

PHASE II STUDY OF T-CELL DEPLETED ALLOGENEIC PERIPHERAL
BLOOD STEM CELL TRANSPLANTATION IN PATIENTS WITH METASTATIC BREAST
CANCER Open
Protocol
PROTOCOL IDS: NCI-00-C-0119; NCI-1027
STUDY CONTACT:
Michael Bishop, Ph: 301-435-2764
Medicine Branch
Bethesda, Maryland

PHASE IV STUDY OF LETROZOLE AS FIRST-LINE THERAPY
IN POST-MENOPAUSAL WOMEN WITH METASTATIC BREAST CANCER PREVIOUSLY
TREATED WITH TAMOXIFEN Open
Protocol
PROTOCOL IDS: NOVARTIS-CFEM345A-US10
STUDY CONTACT:
Mildred Ortu Kowalski, Chair Ph: 973-781-5943
Novartis Pharmaceuticals Corporation

RANDOMIZED STUDY OF VINORELBINE COMBINED WITH CHRONOMODULATED
FLUOROURACIL IN PREVIOUSLY TREATED WOMEN WITH METASTATIC BREAST
CANCER Open
Protocol
PROTOCOL ID: EORTC-05971
STUDY CONTACT:
Bruno Coudert, Ph: 03-80-73-75-28
Centre de Lute Contre le Cancer,
Georges-Francois Leclerc
Dijon, France
EORTC Chronotherapy Group

PHASE III RANDOMIZED STUDY OF PACLITAXEL WITH OR
WITHOUT GEMCITABINE IN WOMEN WITH UNRESECTABLE, LOCALLY RECURRENT,
OR METASTATIC BREAST CANCER Open
Protocol
PROTOCOL ID: LILLY-B9E-MC-JHQG
STUDY CONTACTS:
Furhan Yunus, Chair, Ph: 901-725-1785
Eli Lilly and Company
John M. Waples, Ph: 256-551-6546
Comprehensive Cancer Institute of Huntsville
Huntsville, Alabama

PHASE III RANDOMIZED COMPARISON OF HIGH DOSE CHEMOTHERAPY
PLUS FILGRASTIM TO FILGRASTIM FOR MOBI-LIZATION OF PERIPHERAL BLOOD
STEM CELLS FOR AUTOLOGOUS TRANSPLANTATION FOR PATIENTS WITH RESPONSIVE
METASTATIC
BREAST CANCER OR HIGH RISK STAGE II AND III PATIENTS Open
Protocol
PROTOCOL IDS: MDA-DM-95047; NCI-G96-1014
STUDY CONTACT:
James Gajewski, Ph: 713-745-1644
University of Texas - MD Anderson Cancer Center
Houston, Texas

PHASE III RANDOMIZED STUDY OF DOCETAXEL IN PATIENTS
WITH METASTATIC BREAST CANCER Open
Protocol
PROTOCOL IDS: MDA-ID-99242; NCI-1691;
AVENTIS-MDA-ID-99242
STUDY CONTACT:
Edgardo Rivera, Chair, Ph: 713-792-2817
University of Texas - MD Anderson Cancer Center
Houston, Texas

PHASE II STUDY OF PS-341 IN PATIENTS WITH METASTATIC
BREAST CANCER Open
Protocol
PROTOCOL IDS: MDA-ID-00308
STUDY CONTACT:
Massimo Cristofanilli, Ph: 713-792-2817
University of Texas - MD Anderson Cancer Center
Houston, Texas, U.S.A.

PHASE II STUDY OF OXALIPLATIN IN WOMEN WITH ADVANCED
OR METASTATIC BREAST CANCER FOLLOWING FAILURE OF ANTHRACYCLINE/TAXANE
BASED CHEMOTHERAPY
Open Protocol
PROTOCOL ID: EORTC-16001
STUDY CONTACT:
Pierre Fumoleau, Ph: 33-2-40-67-99-00
CRLCC Nantes - Atlantique
Nantes-Saint Herblain, France

PHASE II RANDOMIZED PILOT STUDY OF CAPECITABINE IN
WOMEN WITH ADVANCED OR METASTATIC BREAST CANCER Open
Protocol
PROTOCOL IDS: PHARMATECH-XEL-154,
PHARMATECH-20010330, ROCHE-PHARMATECH-XEL-154
STUDY CONTACT:
Jane Del Carlo, Chair Ph: 720-917-7453
Pharmatech Oncology
Back | Top of Page

|



