
 |

Select Excerpts from the Discussion
Controversies in adjuvant hormonal therapy
Track 22
![]() DR LOVE: Hy, how are you approaching postmenopausal patients who
have received prior adjuvant tamoxifen? How long can a patient be off
tamoxifen for you to be comfortable starting an aromatase inhibitor
— three years, four years, even five years after tamoxifen?
DR LOVE: Hy, how are you approaching postmenopausal patients who
have received prior adjuvant tamoxifen? How long can a patient be off
tamoxifen for you to be comfortable starting an aromatase inhibitor
— three years, four years, even five years after tamoxifen?
![]() DR MUSS: Five years may be pushing it, although patients have had late
relapses at that point. One of the MA17 papers evaluated hazard ratios over
time. In MA17, patients randomly assigned to the placebo arm had the option
of receiving letrozole after the trial was unblinded.
DR MUSS: Five years may be pushing it, although patients have had late
relapses at that point. One of the MA17 papers evaluated hazard ratios over
time. In MA17, patients randomly assigned to the placebo arm had the option
of receiving letrozole after the trial was unblinded.
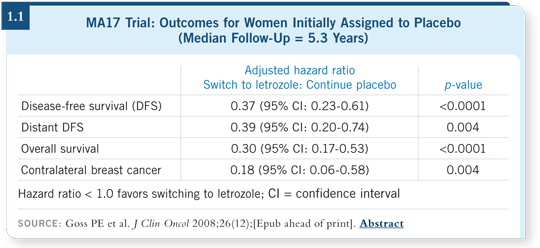
Paul Goss has published those data, which are hard to evaluate for survival because patients weren’t randomly assigned to the treatment, but they seem to show that starting letrozole even several years later may be beneficial (Goss 2008; [1.1]).
That being said, it’s a proportional benefit. In an elderly person with a low-grade, node-negative tumor, I don’t believe it will be especially helpful. Judy Ann Chapman showed some of the causes of death in MA17, and a majority of those patients will die of non-breast cancer causes (Chapman 2008). For a younger postmenopausal patient with node-positive disease who is in good health and has not received any other therapy for a few years, I believe it’s worthy of consideration.
Track 24
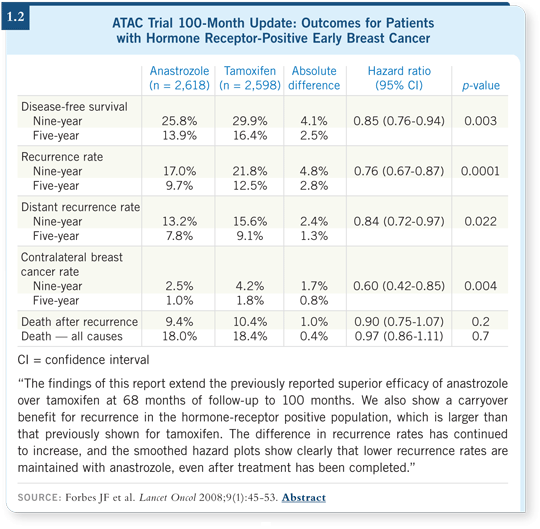
![]() DR LOVE: The recent 100-month update of the ATAC trial (1.2) showed
an increased carryover benefit in years five through nine of anastrozole
compared to tamoxifen for recurrence. Cliff, what’s your current view of
these data?
DR LOVE: The recent 100-month update of the ATAC trial (1.2) showed
an increased carryover benefit in years five through nine of anastrozole
compared to tamoxifen for recurrence. Cliff, what’s your current view of
these data?
![]() DR HUDIS: Frequently, the reason to administer adjuvant therapy or to choose
one regimen over another regimen is to improve survival. However, we make
many choices that do not relate to survival but rather are made for other
concrete reasons, such as better quality of life associated with no recurrence or
administering a less toxic drug. The progression-free survival results are still
being discussed as suggestive of an overall survival advantage, although one
may not exist.
DR HUDIS: Frequently, the reason to administer adjuvant therapy or to choose
one regimen over another regimen is to improve survival. However, we make
many choices that do not relate to survival but rather are made for other
concrete reasons, such as better quality of life associated with no recurrence or
administering a less toxic drug. The progression-free survival results are still
being discussed as suggestive of an overall survival advantage, although one
may not exist.
![]() DR CARLSON: I believe that progression-free survival is an important
endpoint in the adjuvant setting. Most of the treatment options for a woman
who has recurrent breast cancer have significant toxicities, so progression-free
survival in the absence of a clear-cut survival signal is important.
DR CARLSON: I believe that progression-free survival is an important
endpoint in the adjuvant setting. Most of the treatment options for a woman
who has recurrent breast cancer have significant toxicities, so progression-free
survival in the absence of a clear-cut survival signal is important.
Track 18
![]() DR LOVE: Ian, how do you think the data from the clinical trials of
fulvestrant in metastatic disease might influence the study of it in the
adjuvant setting?
DR LOVE: Ian, how do you think the data from the clinical trials of
fulvestrant in metastatic disease might influence the study of it in the
adjuvant setting?
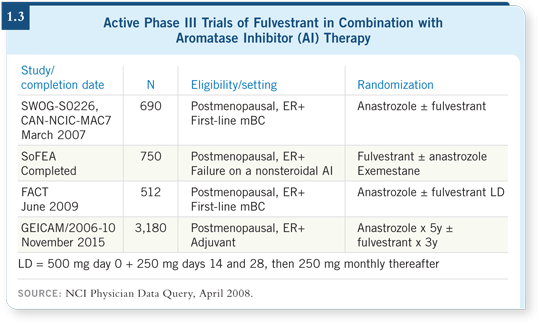
![]() PROF SMITH: For patients with metastatic breast cancer who have failed a
nonsteroidal aromatase inhibitor, the SoFEA trial is comparing the steroidal
aromatase inhibitor exemestane to fulvestrant alone or fulvestrant with
anastrozole, a nonsteroidal aromatase inhibitor (1.3).
PROF SMITH: For patients with metastatic breast cancer who have failed a
nonsteroidal aromatase inhibitor, the SoFEA trial is comparing the steroidal
aromatase inhibitor exemestane to fulvestrant alone or fulvestrant with
anastrozole, a nonsteroidal aromatase inhibitor (1.3).
If the SoFEA trial were positive and reported an additional gain for fulvestrant either after a nonsteroidal aromatase inhibitor or in combination with a nonsteroidal aromatase inhibitor, then that would support an adjuvant trial. My instinct is that if a benefit occurs, it probably will be with the combination. However, the comparative studies of fulvestrant versus tamoxifen (Howell 2004) or the aromatase inhibitors (Howell 2005) have demonstrated equivalency and not superiority.
![]() DR GRALOW: SWOG-S0226, which is accruing well, is an ongoing trial in
the metastatic setting of anastrozole with or without fulvestrant. Crossover
from the anastrozole-alone arm to fulvestrant is strongly encouraged (1.3).
DR GRALOW: SWOG-S0226, which is accruing well, is an ongoing trial in
the metastatic setting of anastrozole with or without fulvestrant. Crossover
from the anastrozole-alone arm to fulvestrant is strongly encouraged (1.3).
Evaluating patients for treatment with adjuvant trastuzumab
Tracks 28-29
![]() DR LOVE: Cliff, what are your thoughts on the selection of adjuvant
therapy for an older woman with, for example, a 2-cm, node-negative,
hormone receptor-negative, HER2-positive breast tumor?
DR LOVE: Cliff, what are your thoughts on the selection of adjuvant
therapy for an older woman with, for example, a 2-cm, node-negative,
hormone receptor-negative, HER2-positive breast tumor?
![]() DR HUDIS: A woman like that would have been eligible for the randomized
Intergroup trastuzumab trial, and I would treat her conventionally with
AC
DR HUDIS: A woman like that would have been eligible for the randomized
Intergroup trastuzumab trial, and I would treat her conventionally with
AC![]() taxane/trastuzumab, if she were interested and willing.
taxane/trastuzumab, if she were interested and willing.
![]() DR GRALOW: The studies support an anthracycline-and taxane-containing
regimen that is a little more aggressive than I want to administer. However,
docetaxel/carboplatin/trastuzumab (TCH) is a tough regimen. Even though
you might have less cardiotoxicity, I believe that you trade it for some other
toxicities.
DR GRALOW: The studies support an anthracycline-and taxane-containing
regimen that is a little more aggressive than I want to administer. However,
docetaxel/carboplatin/trastuzumab (TCH) is a tough regimen. Even though
you might have less cardiotoxicity, I believe that you trade it for some other
toxicities.
I would try to enroll an older patient in our Phase II trial of weekly paclitaxel/trastuzumab, but I don’t have data to do that off study. Off study, I would
probably use AC![]() weekly paclitaxel with trastuzumab.
weekly paclitaxel with trastuzumab.
![]() DR MACKEY: Off study, I would offer this woman TCH. I believe that the
anthracyclines are a major confounding problem with the cardiotoxicity that
we see with trastuzumab. I have found that TCH is reasonably well tolerated.
DR MACKEY: Off study, I would offer this woman TCH. I believe that the
anthracyclines are a major confounding problem with the cardiotoxicity that
we see with trastuzumab. I have found that TCH is reasonably well tolerated.
![]() DR ROBERT: Like John, I was involved with BCIRG 006, and we’ve
prescribed a fair amount of TCH. If the patients had HER2-negative disease,
I would consider four cycles of docetaxel/cyclophosphamide. For patients with
HER2-positive tumors at lower risk, I believe we find some comfort in using
less chemotherapy. So off study I would administer TCH, but I wouldn’t be
wedded to six cycles.
DR ROBERT: Like John, I was involved with BCIRG 006, and we’ve
prescribed a fair amount of TCH. If the patients had HER2-negative disease,
I would consider four cycles of docetaxel/cyclophosphamide. For patients with
HER2-positive tumors at lower risk, I believe we find some comfort in using
less chemotherapy. So off study I would administer TCH, but I wouldn’t be
wedded to six cycles.
![]() DR LOVE: Do you use growth factors?
DR LOVE: Do you use growth factors?
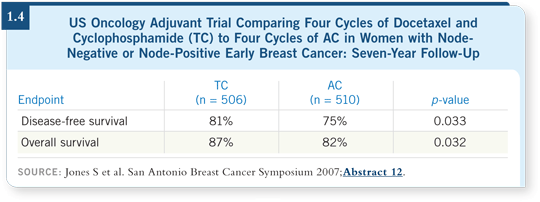
![]() DR ROBERT: For an older patient, I would use growth factors because some
patients become profoundly neutropenic. It is interesting, although not well
known, that in the US Oncology adjuvant trial of TC versus AC, prophylactic
antibiotics were used routinely (Jones 2006; [1.4]).
DR ROBERT: For an older patient, I would use growth factors because some
patients become profoundly neutropenic. It is interesting, although not well
known, that in the US Oncology adjuvant trial of TC versus AC, prophylactic
antibiotics were used routinely (Jones 2006; [1.4]).
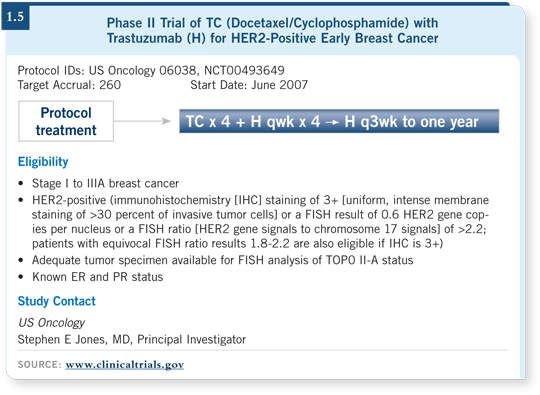
That is something that everyone needs to be aware of because if not, patients may run into problems. I am more comfortable using a nonanthracycline regimen, and we are conducting a trial at US Oncology evaluating docetaxel/cyclophosphamide concomitantly with trastuzumab (1.5).
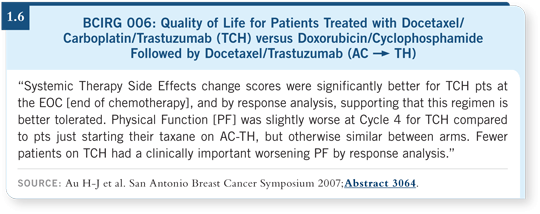
![]() DR MACKEY: We conducted a quality-of-life analysis in BCIRG 006. If
anything, it favored TCH over AC
DR MACKEY: We conducted a quality-of-life analysis in BCIRG 006. If
anything, it favored TCH over AC![]() TH. The patient-reported toxicities
and quality of life were superior with TCH in some components. The global quality-of-life index that was used did not show a statistically significant
difference except at one time point. The trend throughout the entire trial,
however, was in favor of TCH over AC
TH. The patient-reported toxicities
and quality of life were superior with TCH in some components. The global quality-of-life index that was used did not show a statistically significant
difference except at one time point. The trend throughout the entire trial,
however, was in favor of TCH over AC![]() TH (Au 2007; [1.6]).
TH (Au 2007; [1.6]).
Tracks 34-38
![]() DR LOVE: Soon, can you summarize your work on the relation of HER2
status to the benefit derived from adjuvant trastuzumab?
DR LOVE: Soon, can you summarize your work on the relation of HER2
status to the benefit derived from adjuvant trastuzumab?
![]() DR PAIK: It is expected that if HER2 is measured correctly, the assay would
provide linear prediction of benefit. The bottom line is that in the adjuvant
setting, there is no perfectly linear assay to demonstrate trastuzumab response,
including degree of FISH amplification. If the best assay methodology fails to
demonstrate linear correlation and any interaction, then we may have to look for alternative mechanisms of action. As there is no proof either way, a validation
study is needed.
DR PAIK: It is expected that if HER2 is measured correctly, the assay would
provide linear prediction of benefit. The bottom line is that in the adjuvant
setting, there is no perfectly linear assay to demonstrate trastuzumab response,
including degree of FISH amplification. If the best assay methodology fails to
demonstrate linear correlation and any interaction, then we may have to look for alternative mechanisms of action. As there is no proof either way, a validation
study is needed.
![]() DR PEREZ: My initial reaction to the data was, “It doesn’t make any sense.”
This is a targeted drug that has revolutionized the management of breast
cancer.
DR PEREZ: My initial reaction to the data was, “It doesn’t make any sense.”
This is a targeted drug that has revolutionized the management of breast
cancer.
However, we should take seriously the data being accumulated from NSABPB-31 (Paik 2007), NCCTG-N9831 (Reinholz 2007) and HERA (McCaskill-Stevens 2007) that show a lack of correlation between degree of HER2 gene amplification or HER2 overexpression and benefit from trastuzumab.
Also, we should consider the data from the central testing of NSABP-B-31 (1.7) and NCCTG-N9831 (1.8), by which patients with HER2-negative tumors appear to derive the same hazard ratio benefit from trastuzumab as those with HER2-positive tumors.
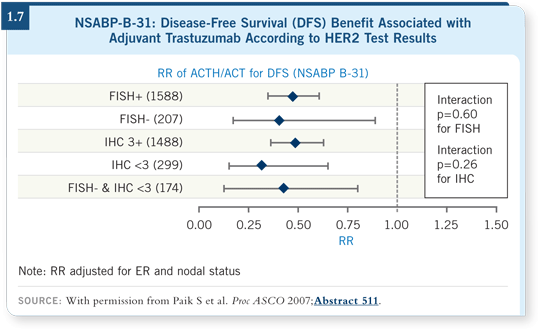
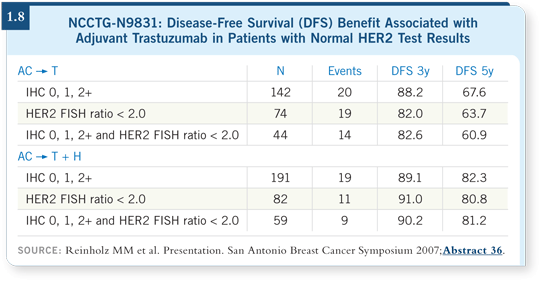
Those specimens were tested by several independent expert pathologists — we’ve gathered the same data. I believe it’s worthy of study because we’re still not curing everyone diagnosed with breast cancer.
![]() DR HUDIS: Keep in mind that all these patients with so-called HER2-negative tumors in the adjuvant trials who seemed to benefit from trastuzumab
were labeled HER2-positive at some point by somebody.
DR HUDIS: Keep in mind that all these patients with so-called HER2-negative tumors in the adjuvant trials who seemed to benefit from trastuzumab
were labeled HER2-positive at some point by somebody.
In CALGB-9840, we explicitly selected patients with “HER2-normal” metastatic breast cancer and randomly assigned them to trastuzumab or not. It’s a negative trial, although to be fair and supportive, a separation of the curves exists visually that may favor the use of trastuzumab (Seidman 2004).
So the question has been asked prospectively for patients with HER2-negative disease, and it’s been asked for lapatinib in patients with HER2-negative disease. A little subtlety exists here in that the patients we keep saying have HER2-negative disease in this adjuvant setting are a subset of patients who were first classified as having HER2-positive disease. I believe that’s distinct from other patients with HER2-negative disease.
![]() DR LOVE: Based on these findings, will a trial be conducted of adjuvant
trastuzumab for patients with HER2-negative disease?
DR LOVE: Based on these findings, will a trial be conducted of adjuvant
trastuzumab for patients with HER2-negative disease?
![]() DR PAIK: Pending further analysis of the NSABP-B-31 central assay data, we
have a concept being reviewed by CTEP that will evaluate chemotherapy with
or without trastuzumab for patients with early breast cancer and low HER2
expression (IHC 1+/2+, FISH-negative).
DR PAIK: Pending further analysis of the NSABP-B-31 central assay data, we
have a concept being reviewed by CTEP that will evaluate chemotherapy with
or without trastuzumab for patients with early breast cancer and low HER2
expression (IHC 1+/2+, FISH-negative).
![]() DR GRALOW: I believe it’s important to study trastuzumab in the HER2-negative population, but I’m not ready to use trastuzumab off study for a
patient who clearly has HER2-negative disease.
I am starting to get calls now that the American Society for Clinical Oncology
(ASCO)/College of American Pathologists (CAP) guidelines have defined an
equivocal range for HER results (Wolff 2007).
DR GRALOW: I believe it’s important to study trastuzumab in the HER2-negative population, but I’m not ready to use trastuzumab off study for a
patient who clearly has HER2-negative disease.
I am starting to get calls now that the American Society for Clinical Oncology
(ASCO)/College of American Pathologists (CAP) guidelines have defined an
equivocal range for HER results (Wolff 2007).
![]() DR WOLFF: The ASCO/CAP guidelines use the term equivocal as part of
an attempt to force further characterization of these specimens. If you have
a specimen with a low FISH ratio that is around two, you should perform
immunohistochemistry. The documents explicitly state that these patients were
eligible for the adjuvant trastuzumab trials. So at this point, the guidelines do
not say that these patients should not be offered trastuzumab (Wolff 2007).
DR WOLFF: The ASCO/CAP guidelines use the term equivocal as part of
an attempt to force further characterization of these specimens. If you have
a specimen with a low FISH ratio that is around two, you should perform
immunohistochemistry. The documents explicitly state that these patients were
eligible for the adjuvant trastuzumab trials. So at this point, the guidelines do
not say that these patients should not be offered trastuzumab (Wolff 2007).
Based on the data we have from the prospective studies, in which patients with a FISH ratio of two and higher or with IHC 3+ disease were eligible, I believe it is appropriate to offer trastuzumab to those patients.
![]() DR LOVE: Should we use the entry criteria from the adjuvant trials when
deciding who should receive adjuvant trastuzumab?
DR LOVE: Should we use the entry criteria from the adjuvant trials when
deciding who should receive adjuvant trastuzumab?
![]() DR WOLFF: That is exactly what the guidelines state. The guidelines cannot
be used by anybody as support to deny trastuzumab to these patients.
DR WOLFF: That is exactly what the guidelines state. The guidelines cannot
be used by anybody as support to deny trastuzumab to these patients.
![]() DR CARLSON: I believe two underlying assumptions may be incorrect, and
if they are discarded, the responsiveness of this HER2-negative subset may
make more sense. First, the so-called HER2-negative tumors do have HER2
present on the cell surface. These tumors are not technically HER2-negative,
but rather they are HER2-low expressing or HER2-normal. So in fact, a
target may still exist for trastuzumab.
DR CARLSON: I believe two underlying assumptions may be incorrect, and
if they are discarded, the responsiveness of this HER2-negative subset may
make more sense. First, the so-called HER2-negative tumors do have HER2
present on the cell surface. These tumors are not technically HER2-negative,
but rather they are HER2-low expressing or HER2-normal. So in fact, a
target may still exist for trastuzumab.
The other assumption is that if high levels of HER2 are present, then there’s a lot of signal transduction, and trastuzumab will interfere with that signal transduction. However, many data indicate that trastuzumab has other mechanisms of action. It may be that inhibiting signal transduction is not the biologically active mechanism in the adjuvant setting. Indeed, a fair amount of data suggests that it is immunologic.
Clinical trials incorporating adjuvant bevacizumab
Tracks 42-43
![]() DR LOVE: Can you discuss the rationale for using docetaxel/carboplatin/trastuzumab (TCH) as the base regimen for the BETH trial evaluating
bevacizumab in patients with HER2-positive tumors?
DR LOVE: Can you discuss the rationale for using docetaxel/carboplatin/trastuzumab (TCH) as the base regimen for the BETH trial evaluating
bevacizumab in patients with HER2-positive tumors?
![]() DR MACKEY: In 2006, when we presented the second analysis of the BCIRG
006 trial at the San Antonio Breast Cancer Symposium, the findings related to
the critical toxicities associated with an anthracycline-containing trastuzumab
regimen were a bit of a “lone cry in the woods” (Slamon 2006; [1.9]).
DR MACKEY: In 2006, when we presented the second analysis of the BCIRG
006 trial at the San Antonio Breast Cancer Symposium, the findings related to
the critical toxicities associated with an anthracycline-containing trastuzumab
regimen were a bit of a “lone cry in the woods” (Slamon 2006; [1.9]).
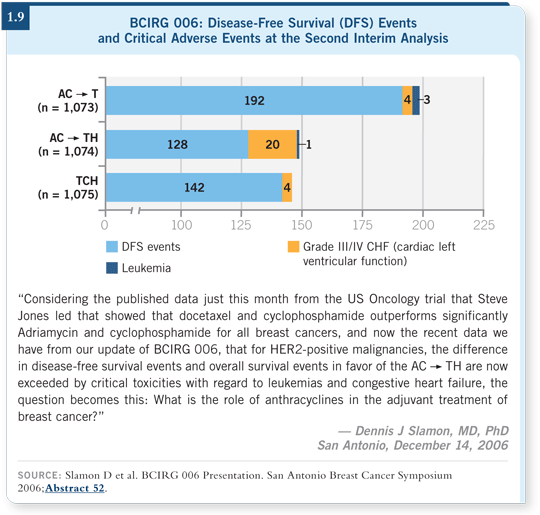
However, over time some interesting data have come from a number of sources suggesting that there is something to this HER2-anthracycline interaction and that the cardiotoxicity and leukemogenecity are perhaps real concerns, particularly for women who are older than 65 years of age.
In addition, we’re all hopeful that the adjuvant anti-angiogenic strategies will provide the way forward in the population with HER2-positive disease. This would require a relatively noncardiotoxic background because all the currently available agents carry some potential risk for exacerbating cardiotoxicity.
![]() DR LOVE: Chuck, can you discuss the BETH trial?
DR LOVE: Chuck, can you discuss the BETH trial?
![]() DR GEYER: The NSABP, by participating in the BETH trial and choosing
TCH as the standard arm, is answering yes to its use (1.10). In reviewing
the efficacy data from BCIRG 006, it seems impossible to truly differentiate
between TCH and AC
DR GEYER: The NSABP, by participating in the BETH trial and choosing
TCH as the standard arm, is answering yes to its use (1.10). In reviewing
the efficacy data from BCIRG 006, it seems impossible to truly differentiate
between TCH and AC![]() TH at this point (Slamon 2006; [1.9]).
TH at this point (Slamon 2006; [1.9]).
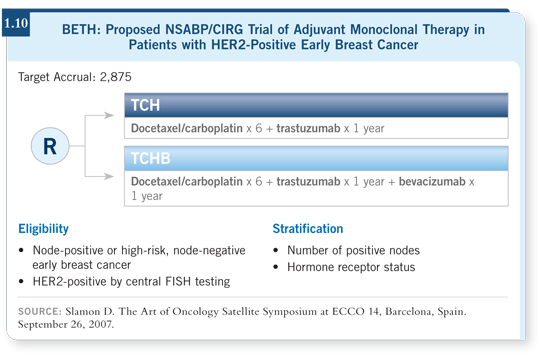
The curve for disease-free survival is slightly higher with the anthracycline-containing regimen, but statistically the confidence intervals overlap. In addition, the nonanthracycline-containing arm yields fewer serious toxicities. So far, it clearly has less cardiotoxicity and possibly fewer cases of leukemia (Slamon 2006; [1.9]). When I talk to patients in clinical practice, I explain both regimens, and I find patients generally opt for the regimen that seems to be less cardiotoxic. Now maybe I’m biasing my presentation, but I provide them the information and the safety factor seems to be swaying my patients.
Track 10
![]() DR LOVE: Julie, can you discuss the data presented at the 2007 San
Antonio Breast Cancer Symposium on the cardiac safety of dose-dense
AC followed by nanoparticle albumin-bound (nab) paclitaxel combined
with bevacizumab?
DR LOVE: Julie, can you discuss the data presented at the 2007 San
Antonio Breast Cancer Symposium on the cardiac safety of dose-dense
AC followed by nanoparticle albumin-bound (nab) paclitaxel combined
with bevacizumab?
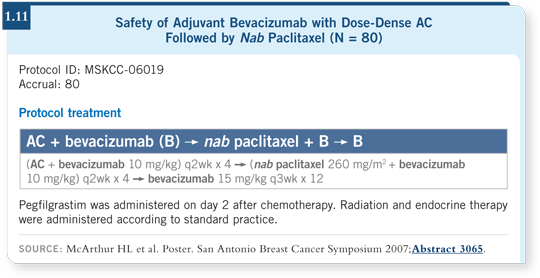
![]() DR GRALOW: This was an adjuvant trial with 80 patients with HER2-negative, early-stage breast cancer and normal LVEF. Patients received dose-dense
AC with growth factor support followed by dose-dense nab paclitaxel at
260 mg/m2 every two weeks times four with growth factor support.
DR GRALOW: This was an adjuvant trial with 80 patients with HER2-negative, early-stage breast cancer and normal LVEF. Patients received dose-dense
AC with growth factor support followed by dose-dense nab paclitaxel at
260 mg/m2 every two weeks times four with growth factor support.
Bevacizumab at 10 mg/kg was administered concurrently with the chemotherapy every other week and then switched to 15 mg/kg every three weeks to complete one year (McArthur 2007; [1.11]). Patients did not exhibit symptomatic left ventricular dysfunction. However, three out of 80 patients, or four percent, had uncontrolled hypertension. I believe that as we administer bevacizumab in the long term, we will struggle with hypertension the most as an obvious toxicity.
Determining which patients are candidates for adjuvant chemotherapy
Tracks 46-48
![]() DR LOVE: Julie, do you believe the Oncotype DX™ assay can be utilized
to determine whether patients with node-positive breast cancer should
receive adjuvant chemotherapy?
DR LOVE: Julie, do you believe the Oncotype DX™ assay can be utilized
to determine whether patients with node-positive breast cancer should
receive adjuvant chemotherapy?
![]() DR GRALOW: For a select group of patients with positive nodes, I do. I have
ordered it a couple of times, such as for patients with low nodal burden. I am
not ready to order it for a patient with 10 positive nodes and use it to trump
the other features of their disease. For a patient who I believe is highly sensitive
to endocrine therapy and has a little nodal disease, I would consider it.
DR GRALOW: For a select group of patients with positive nodes, I do. I have
ordered it a couple of times, such as for patients with low nodal burden. I am
not ready to order it for a patient with 10 positive nodes and use it to trump
the other features of their disease. For a patient who I believe is highly sensitive
to endocrine therapy and has a little nodal disease, I would consider it.
![]() DR HUDIS: I recently saw a 78-year-old woman in otherwise good health
with a 2-cm, ER-positive (100 percent), PR-negative (zero), HER2-normal
tumor and a single positive lymph node out of 14. Her attitude was that she
would accept chemotherapy if it was needed. That was one of the first times
I’ve ordered an Oncotype DX assay for a patient with node-positive disease.
DR HUDIS: I recently saw a 78-year-old woman in otherwise good health
with a 2-cm, ER-positive (100 percent), PR-negative (zero), HER2-normal
tumor and a single positive lymph node out of 14. Her attitude was that she
would accept chemotherapy if it was needed. That was one of the first times
I’ve ordered an Oncotype DX assay for a patient with node-positive disease.
Conversely, I believe it is risky to withhold adjuvant chemotherapy from cohorts of patients for whom it’s been recommended, especially among those with node-positive disease, based on relatively small absolute numbers of events. When the patient is on the fence about receiving chemotherapy and you’re having a discussion, that’s a different situation.
![]() DR MUSS: It is reasonable to use Oncotype DX for a patient with one positive
node who is on the fence about whether or not to take chemotherapy. Like Cliff,
I would be nervous about using this routinely, except for an occasional patient.
DR MUSS: It is reasonable to use Oncotype DX for a patient with one positive
node who is on the fence about whether or not to take chemotherapy. Like Cliff,
I would be nervous about using this routinely, except for an occasional patient.
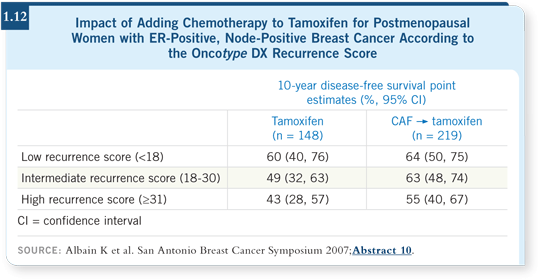
Also, these studies we’re discussing have notably small numbers of patients (Goldstein 2007; Albain 2007; [1.12]). We’re pruning them down and ending up with 200 patients in a subset analysis. We need to be cautious.
EDITOR
Neil Love, MD
TOPICS
Treatment of Early Breast Cancer
- Select publications
Treatment of Metastatic Breast Cancer
- Select publications
Breast Cancer Update - Think Tank:
A CME Audio Series and Activity