
 |
||||||||
![]()

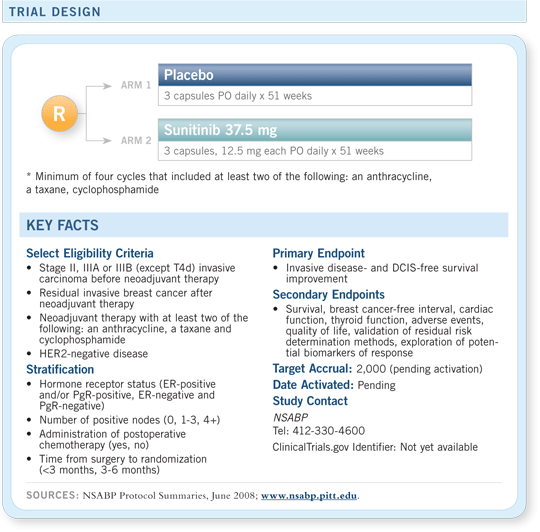
NSABP-B-45
A Phase III clinical trial comparing adjuvant sunitinib malate to placebo after
neoadjuvant chemotherapy*
![]()
“Clinicians and patients often wonder whether additional chemotherapy should be given in the adjuvant setting after preoperative chemotherapy treatment. To date, no trial has shown that additional chemotherapy after a modern preoperative chemotherapy (generally anthracycline- and taxane-based therapy) improves outcome...
Although there is no evidence that more chemotherapy will be of value, it is also fully recognized that this clinical situation [residual invasive breast cancer] is one that arises frequently in clinical practice, given that the majority of patients treated with preoperative therapy do not achieve a complete pathologic response. Outside of a clinical trial, the use of additional chemotherapy after a standard course of treatment should be discouraged. Within the context of a clinical trial, a variety of approaches could be tested in this setting, including the use of non–cross-resistant chemotherapy, angiogenesis inhibitors, high-dose bisphosphonates, vaccines, and a variety of other biologic therapies.”
— Gralow JR et al.
J Clin Oncol 2008;26:814-9.

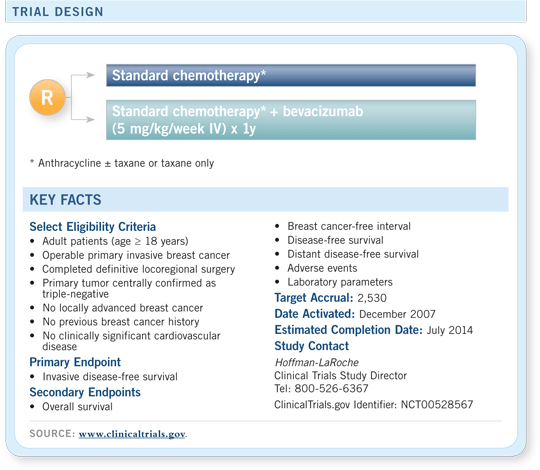
BEATRICE
A randomized, Phase III, open-label study of bevacizumab as adjuvant therapy for
triple-negative disease
![]()
“There is basic and clinical evidence supporting the important role of angiogenesis in breast cancer. Although no specific overexpression of VEGF has been described in BLBC, there has been a morphologic description that such tumors have vascularity, potentially justifying VEGF as a suitable target in TNBC.
These findings are further supported by the results of a randomized phase III trial led by the Eastern Cooperative Oncology Group. E2100 compared paclitaxel with or without bevacizumab as front-line therapy in patients with metastatic breast cancer. Only 1% of patients were HER2-positive.
In a subset analysis, the addition of bevacizumab benefited several groups, including patients with ER-negative and PR-negative tumors. In this cohort, progression-free survival improved in the combination arm (8.8 months vs 4.6 months, hazard ratio 0.53).
With this encouraging clinical activity in the metastatic setting, bevacizumab is being tested in patients with early stage TNBC.”
[BLBC = basal-like breast cancer; TNBC = triplenegative breast cancer]
— Wasserman EJ, Tan AR
ASCO Educational Book, 2008

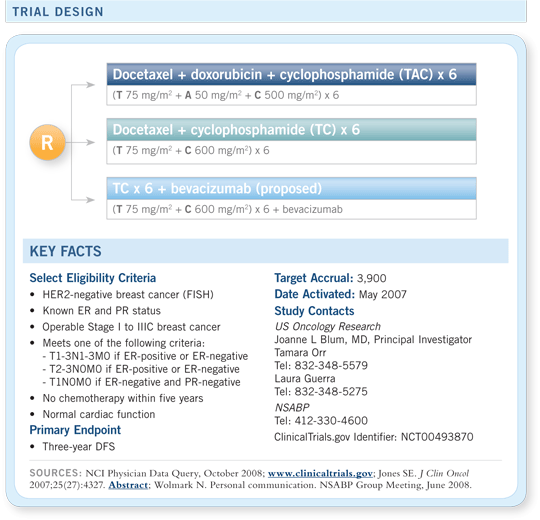
US Oncology “TIC-TAC-TOE”
NSABP proposed amendment to US Oncology 06090
A Phase III trial of adjuvant TC versus TAC versus TC/bevacizumab
![]()
“Sarah Cannon and US Oncology are evaluating six cycles of TAC versus six cycles of TC, but will that be a definitive trial? The target sample size is 2,000 patients, and the study has 80 percent power to detect a 3.4 percent absolute difference in disease-free survival in favor of the anthracycline. What if the difference is only three percent and the p-value is 0.08? What conclusions will we derive?
So the NSABP, along with Steve Jones and US Oncology, would like to fold that trial into the ‘TIC-TAC-TOE’ trial, or the 3T trial, in which we’re comparing TAC to TC to TC/bevacizumab in 3,900 patients.
We hope the last arm will determine whether bevacizumab on a nonanthracycline template can add benefit, and it will increase the sample size for the pairwise comparison of TAC to TC to approximately 3,600, which would provide more power to determine the value of an anthracycline or the lack thereof in a HER2-negative cohort.”
— Norman Wolmark, MD
Breast Cancer Update Issue 5, 2008

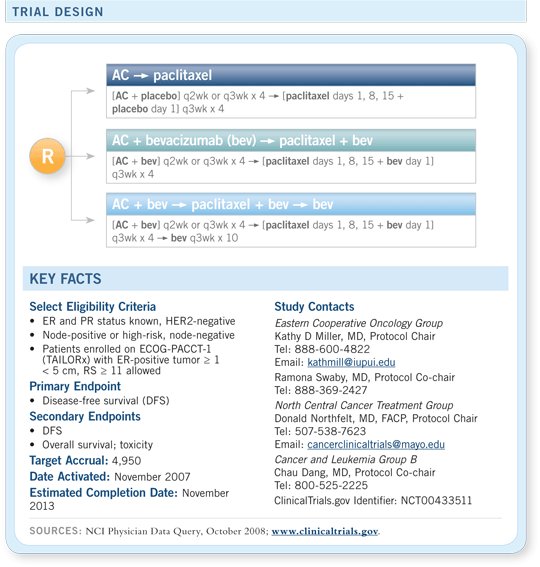
ECOG-E5103
A Phase III study of adjuvant AC followed by paclitaxel with or without bevacizumab
![]()
“I believe that bevacizumab will be effective in the adjuvant setting, but hypotheses with evidence exist on both sides of the question. Angiogenesis may be regarded as one of the earliest events that a tumor cell must accomplish. We see evidence of angiogenesis even in DCIS, in which the tumors are not yet invasive. It is an early phenomenon, which suggest that agents like bevacizumab might be more effective earlier in the course of the disease, which would bring you into the adjuvant setting. I also have questions about the duration of therapy. We administer bevacizumab for two durations in E5103: approximately six months and approximately one year. Perhaps that’s not long enough. Perhaps you need chronic therapy, not to eliminate microscopic disease but rather to keep it from growing. If we remove that foot from the brake, we may prolong time to progression but perhaps not prevent recurrence or change overall survival.”
— Kathy D Miller, MD
Breast Cancer Update Issue 5, 2008

ALTTO (BIG 2-06)
A randomized, multicenter, open-label, Phase III study of adjuvant lapatinib, trastuzumab,
their sequence and their combination in patients with HER2-positive primary breast cancer


NSABP/CIRG BETH
Chemotherapy and trastuzumab with or without bevacizumab as treatment for patients with
HER2-positive early breast cancer

![]()
“We know that HER2 is an upstream regulator of VEGF production. That has been shown definitively both in cell lines and in the clinic. A woman who has HER2-positive breast cancer simply has more VEGF in her tumor. In some lovely preclinical modeling that was presented in Nature a few years ago, the HER2-positive tumors were considerably more vascular than the HER2-negative tumors. From a clinical standpoint, these tumors have a higher microvessel density. More importantly, VEGF expression is a clear regulator of outcome. In a study conducted by Gottfried Konecny at UCLA, the tumors with the worst performance were the ones that were both HER2-positive and VEGF-positive, so that combination of HER2 positivity and VEGF overexpression appears to be clinically important.”
— George W Sledge Jr, MD
Breast Cancer Update Issue 6, 2007
EDITOR'S NOTE
Bail Us Out
Neil Love, MD
CLINICAL TRIALS
Neoadjuvant Therapy
Adjuvant Therapy
Metastatic Disease
Breast Cancer
Clinical Trials Guide:
A CME Audio Series and Activity