|
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR BURSTEIN: More and more, we are splintering breast cancer into several different diseases, so most of the clinical trials moving forward are focusing on biologically defined subsets of patients with breast cancer. We will see specific studies for hormone receptor-positive disease, for HER2-overexpressing disease and for triple-negative or basal-like disease. I believe this is reasonable because the questions of most interest are probably different in each of those patient populations.
DR BURSTEIN: More and more, we are splintering breast cancer into several different diseases, so most of the clinical trials moving forward are focusing on biologically defined subsets of patients with breast cancer. We will see specific studies for hormone receptor-positive disease, for HER2-overexpressing disease and for triple-negative or basal-like disease. I believe this is reasonable because the questions of most interest are probably different in each of those patient populations.
![]() DR LOVE: You wrote a number of articles — prior to the publication of the adjuvant trastuzumab trials — that said that in addition to the likelihood that the trastuzumab data were going to be positive, the data would most likely affect how we treat patients with HER2-negative disease and the trials that those patients enter.
DR LOVE: You wrote a number of articles — prior to the publication of the adjuvant trastuzumab trials — that said that in addition to the likelihood that the trastuzumab data were going to be positive, the data would most likely affect how we treat patients with HER2-negative disease and the trials that those patients enter.
![]() DR BURSTEIN: That’s right, and that’s even truer at this time. What we thought we knew about treating with adjuvant chemotherapy, for instance, was all derived from trials that included patients with all different types of breast cancer.
DR BURSTEIN: That’s right, and that’s even truer at this time. What we thought we knew about treating with adjuvant chemotherapy, for instance, was all derived from trials that included patients with all different types of breast cancer.
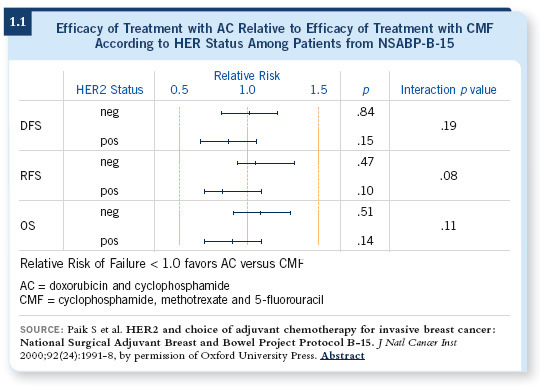
If you carefully review the role of anthracyclines, you see that a lot of retrospective data (Paik 1998, 2000) suggest that most of the benefit of anthracycline therapy is in HER2-overexpressing breast cancer (1.1). Once you take those patients with HER2-positive disease out of the general mix, it’s not as clear that anthracyclines are critically important.
Similarly, a lot of retrospective work now suggests that the benefits associated with the major tweaks of chemotherapy are mostly observed in hormone receptor-negative breast cancer. For hormone receptor-positive breast cancer, it is not as clear that those are major advances (Berry 2006), so the idea of separating breast cancer into different subsets makes sense, but it does present challenges.
The first is that the creation of subsets dilutes the patient population base, so instead of rapid accrual to studies that require thousands of patients to enroll, we will need more carefully selected patients.
Second, the identification of subsets puts a high priority on the quality of testing tumors. Obviously, if you’re going to make clinical decisions based on anything such as hormone receptor status, HER2 status, or Oncotype DX™ and genomic assays, you must know the reproducibility of the testing and the quality control. That will be a challenge for community oncologists in the United States.
No fewer than three expert panels are currently convened to discuss HER2 testing. ASCO, the National Comprehensive Cancer Network and the College of American Pathology are all issuing guidelines for HER2 testing. My understanding, although of course the details may change, is that most of these panels will make the same recommendation, namely, that either well-conducted immunohistochemical testing or well-conducted FISH testing is acceptable.
But the key questions are, how good is the specific tester, and what quality control measures does the pathologist use? The panels will demand that pathologists prove they can conduct high-quality testing, or they will be asked to stop HER2 testing. That’s a big change.
If you’re a practicing oncologist who is about to treat a patient for HER2-overexpressing disease based on the results of a certain pathology lab and you don’t know the performance characteristics of that lab, you can’t make a good decision about whether or not HER2 is overexpressed.
![]() DR LOVE: A lot of concern has arisen about HER2 testing because of the excitement about adjuvant trastuzumab, but some observers think that ER testing is in even worse shape.
DR LOVE: A lot of concern has arisen about HER2 testing because of the excitement about adjuvant trastuzumab, but some observers think that ER testing is in even worse shape.
![]() DR BURSTEIN: When it was appreciated five or six years ago that HER2 testing was in many ways mediocre, a lot of educational campaigns and quality initiatives were put forward to address those concerns. It has been shown that you can train people to do a better job, but that same educational process has not happened in ER testing for decades, and that is a significant issue.
DR BURSTEIN: When it was appreciated five or six years ago that HER2 testing was in many ways mediocre, a lot of educational campaigns and quality initiatives were put forward to address those concerns. It has been shown that you can train people to do a better job, but that same educational process has not happened in ER testing for decades, and that is a significant issue.
Regarding the Genomic Health and the Oncotype DX experience, when they start taking sensitive quantitative RNA measurements for genes, including ER and PR, my understanding is that a small fraction — probably five or 10 percent — of cases that are thought to be ER-positive will be ER-negative.
So here is the real lesson, and it is not unique to breast cancer: As soon as you ask the pathology service to tell you more than whether a tumor is present, how big it is and whether nodes are involved, you introduce a level of expertise that not every pathology group will have.
We will need high-quality, high-volume pathology testing for all these new molecular markers that are coming along in all the different tumor types. That will be a challenge for the pathologists. The good ones will rise to that challenge by instituting appropriate quality assurance plans. They will also realize that they can’t do every test well, so they will collaborate with people who may do certain tests better than they do.
Track 5
![]() DR LOVE: What are some of the strategies — specifically focusing on ER-positive disease — you think might reduce recurrence rates and mortality in the future?
DR LOVE: What are some of the strategies — specifically focusing on ER-positive disease — you think might reduce recurrence rates and mortality in the future?
![]() DR BURSTEIN: Work is being done in several important areas. One involves the question about treatment with aromatase inhibitors in the early-stage setting: When and for how long? Well-orchestrated trials are tackling those questions, and there will be chances to update those experiences annually for many years to come.
DR BURSTEIN: Work is being done in several important areas. One involves the question about treatment with aromatase inhibitors in the early-stage setting: When and for how long? Well-orchestrated trials are tackling those questions, and there will be chances to update those experiences annually for many years to come.
Another big question is to figure out, within the group of patients with ER-positive early-stage breast cancer, which women need chemotherapy. A lot of attention has focused on the Oncotype DX (Paik 2004) experience with the NSABP and Soon Paik. I like that general approach. I believe it exemplifies where most of us believe the field should be going.
![]() DR LOVE: Are you using Oncotype in your practice?
DR LOVE: Are you using Oncotype in your practice?
![]() DR BURSTEIN: I use it in my practice, and I find it is very helpful. Patients understand it, and it resonates with other measures in breast cancer pathology. It gives you a quantitative readout. Patients readily understand that this is an extra piece of information that can help us stratify risk and therefore allow them to make a better-informed choice about chemotherapy.
DR BURSTEIN: I use it in my practice, and I find it is very helpful. Patients understand it, and it resonates with other measures in breast cancer pathology. It gives you a quantitative readout. Patients readily understand that this is an extra piece of information that can help us stratify risk and therefore allow them to make a better-informed choice about chemotherapy.
I believe this will remarkably change the playing field for ER-positive breast cancer. Many of these women will no longer be receiving chemotherapy. They simply won’t need it, based on their prognosis. However, we need to refine that.
The Oncotype DX test was developed with tamoxifen-treated patients who received what many would consider old-fashioned chemotherapy. I believe the principles will be the same, but we want to see studies in the adjuvant setting using aromatase inhibitors, and we want to see studies that use different chemotherapy programs, and so on.
![]() DR LOVE: What about patients with node-positive disease?
DR LOVE: What about patients with node-positive disease?
![]() DR BURSTEIN:This approach will be extended to node-positive disease. These ideas are being worked on. Again, the general question is, how can we use genomic-type information and the analysis of multiple gene expression patterns to refine our prognosis and treatment selection? That general strategy is appearing across cancer medicine and specifically in breast cancer, and this choice of which patients with ER-positive disease need chemotherapy is the place where it has been most readily and successfully exploited so far.
DR BURSTEIN:This approach will be extended to node-positive disease. These ideas are being worked on. Again, the general question is, how can we use genomic-type information and the analysis of multiple gene expression patterns to refine our prognosis and treatment selection? That general strategy is appearing across cancer medicine and specifically in breast cancer, and this choice of which patients with ER-positive disease need chemotherapy is the place where it has been most readily and successfully exploited so far.
Track 10
![]() DR LOVE: What are some of the most frequently asked questions you receive from community-based oncologists?
DR LOVE: What are some of the most frequently asked questions you receive from community-based oncologists?
![]() DR BURSTEIN: The biggest question I hear at tumor boards right now is how to approach patients who have small HER2-positive tumors such as the patient with the 7-mm, ER-negative, HER2-positive tumor or the 1.2-cm, ER-positive, HER2-positive tumor.
DR BURSTEIN: The biggest question I hear at tumor boards right now is how to approach patients who have small HER2-positive tumors such as the patient with the 7-mm, ER-negative, HER2-positive tumor or the 1.2-cm, ER-positive, HER2-positive tumor.
We don’t have great data on the outcomes for these women. Our group has proposed, and I believe we’ll put forward, a multicenter trial evaluating trastuzumab with paclitaxel as a treatment regimen for patients at low risk.
We will treat approximately 300-400 patients in what will essentially be a feasibility study to show that if you carefully select the patients at low risk and administer a paclitaxel/trastuzumab combination that should be well tolerated, you have a low risk of recurrence.
We would love to see a huge randomized trial for these women, but that is impractical given the resources and the generally low risk for patients with node-negative disease.
Track 11
![]() DR LOVE: Can you discuss the treatment of patients with triple-negative tumors?
DR LOVE: Can you discuss the treatment of patients with triple-negative tumors?
![]() DR BURSTEIN: We don’t have a targeted agent for these tumors, so the work in this area has been focusing on optimizing chemotherapy. Some trials are evaluating adding products like capecitabine, and some are evaluating platinum-based chemotherapy.
DR BURSTEIN: We don’t have a targeted agent for these tumors, so the work in this area has been focusing on optimizing chemotherapy. Some trials are evaluating adding products like capecitabine, and some are evaluating platinum-based chemotherapy.
Additionally, there is interest in other biological approaches, and probably the one that is furthest along has been to add bevacizumab to the treatment of these patients.
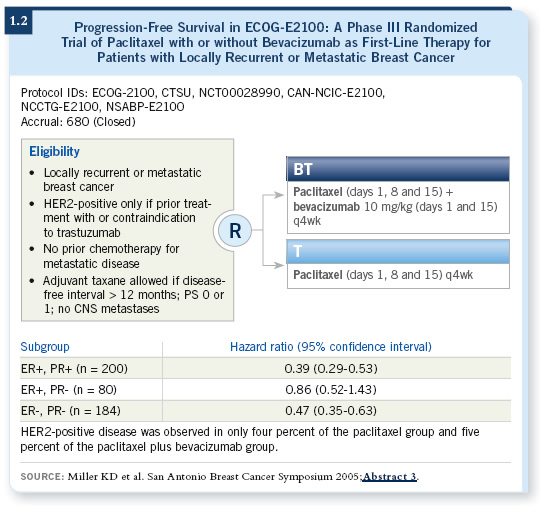
ECOG-E2100 (1.2) indicated that the ER-negative, HER2-negative patients did handsomely with paclitaxel and bevacizumab. So that is a reasonable patient population in which to try optimizing chemotherapy and other biological approaches.
![]() DR LOVE: In general, how do you approach therapy for a woman with visceral metastatic disease that is extensive, symptomatic, associated with poor performance status, triple-negative and chemotherapy naïve?
DR LOVE: In general, how do you approach therapy for a woman with visceral metastatic disease that is extensive, symptomatic, associated with poor performance status, triple-negative and chemotherapy naïve?
![]() DR BURSTEIN: Obviously, we will administer chemotherapy. Most frequently, I use paclitaxel with bevacizumab for patients like that. I find the data from ECOG-E2100 compelling. We can do better than using chemotherapy alone by adding bevacizumab treatment. I like the idea of using a relatively exciting biological therapy. The other point is that few women who walk in the door are chemotherapy naïve at that point.
DR BURSTEIN: Obviously, we will administer chemotherapy. Most frequently, I use paclitaxel with bevacizumab for patients like that. I find the data from ECOG-E2100 compelling. We can do better than using chemotherapy alone by adding bevacizumab treatment. I like the idea of using a relatively exciting biological therapy. The other point is that few women who walk in the door are chemotherapy naïve at that point.
Track 14
![]() DR LOVE: Can you provide an update on trials looking at bevacizumab in the adjuvant setting?
DR LOVE: Can you provide an update on trials looking at bevacizumab in the adjuvant setting?
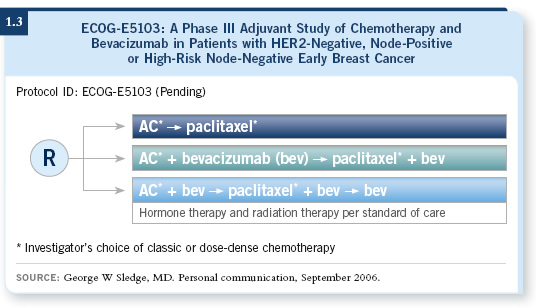
![]() DR BURSTEIN: Bevacizumab is moving to the adjuvant setting. The Intergroup
will be running a trial (1.3) with a design of AC followed by paclitaxel with or without bevacizumab.
DR BURSTEIN: Bevacizumab is moving to the adjuvant setting. The Intergroup
will be running a trial (1.3) with a design of AC followed by paclitaxel with or without bevacizumab.
What makes this exciting is that bevacizumab is an encouraging drug. If you believe the anti-angiogenesis model and the hypothesis that targeting the blood supply will inhibit the growth of tumors, the best setting in which to observe that will be the adjuvant setting because you have micrometastatic disease. It does not have a fully established blood supply already.
If you agree with the Judah Folkman hypothesis, this setting is where you want to evaluate these types of drugs. These clinical trials have the potential to be tremendously beneficial and important.
One needs to conduct the studies, of course, but there is a reasonable chance that the benefits will be even better in the adjuvant setting than in the metastatic setting.
![]() DR LOVE: How do you think the safety and tolerability profile will play out in the adjuvant setting?
DR LOVE: How do you think the safety and tolerability profile will play out in the adjuvant setting?
![]() DR BURSTEIN: I believe it will be feasible to administer the drug. The median time to progression with paclitaxel and bevacizumab in ECOG-E2100 was 11 months, so we know that you can feasibly administer the drug for a long period, but we don’t know about the late side effects.
DR BURSTEIN: I believe it will be feasible to administer the drug. The median time to progression with paclitaxel and bevacizumab in ECOG-E2100 was 11 months, so we know that you can feasibly administer the drug for a long period, but we don’t know about the late side effects.