
 |
||||||||

| Tracks 1-9 | ||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: You just returned from Oxford and the latest trialists meeting. What will the new Oxford Overview analysis tell us about adjuvant chemotherapy?
DR LOVE: You just returned from Oxford and the latest trialists meeting. What will the new Oxford Overview analysis tell us about adjuvant chemotherapy?
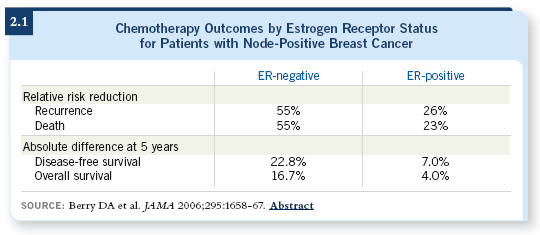
![]() DR RAVDIN: The questions include: How much do taxanes add and what about subsets of patients receiving taxanes? Individual trials have suggested taxanes may be more effective in patients with a few positive nodes than those with many positive nodes. Other trials, notably the analysis of CALGB data by Don Berry and colleagues, have suggested that these agents work well in patients with ER-negative disease but don’t add much for those with ER-positive tumors (Berry 2006; [2.1]). In the Oxford Overview, we were not surprised to see that taxanes improve recurrence-free and overall survival. In a subset analysis of pooled data, both patients with node-negative and node-positive disease appeared to be benefiting.
DR RAVDIN: The questions include: How much do taxanes add and what about subsets of patients receiving taxanes? Individual trials have suggested taxanes may be more effective in patients with a few positive nodes than those with many positive nodes. Other trials, notably the analysis of CALGB data by Don Berry and colleagues, have suggested that these agents work well in patients with ER-negative disease but don’t add much for those with ER-positive tumors (Berry 2006; [2.1]). In the Oxford Overview, we were not surprised to see that taxanes improve recurrence-free and overall survival. In a subset analysis of pooled data, both patients with node-negative and node-positive disease appeared to be benefiting.
![]() DR LOVE: Roughly speaking, what was the relative reduction of risk of recurrence
and death for taxanes versus nontaxanes?
DR LOVE: Roughly speaking, what was the relative reduction of risk of recurrence
and death for taxanes versus nontaxanes?
![]() DR RAVDIN: Roughly 20 percent. The Oxford Overview analysis includes trials with an arm containing a taxane versus one that does not, but it includes a mix of trials. Sometimes the two regimens are identical except for the addition of a taxane. For example, CALGB trial 9344 is AC versus AC with paclitaxel (Henderson 1998). Other trials evaluated a taxane versus some other regimen. The BCIRG 001 trial evaluated TAC versus FAC (Martin 2005).
DR RAVDIN: Roughly 20 percent. The Oxford Overview analysis includes trials with an arm containing a taxane versus one that does not, but it includes a mix of trials. Sometimes the two regimens are identical except for the addition of a taxane. For example, CALGB trial 9344 is AC versus AC with paclitaxel (Henderson 1998). Other trials evaluated a taxane versus some other regimen. The BCIRG 001 trial evaluated TAC versus FAC (Martin 2005).
Estrogen receptor status did not seem to strongly modulate the benefit of taxanes. This isn’t a big surprise because if individual trials don’t find a correlation with ER status, they don’t emphasize it. For instance, the TAC/FAC trial didn’t show a large difference in benefit by ER status (Martin 2005). When all the trials were assessed together, ER itself did not appear to be a predictor of particular benefit from taxanes.
Tracks 4, 6
![]() DR LOVE: In the overall Oxford Overview chemotherapy data, what was the correlation between estrogen receptor status and outcome?
DR LOVE: In the overall Oxford Overview chemotherapy data, what was the correlation between estrogen receptor status and outcome?
![]() DR RAVDIN: This was hotly debated and complicated by the fact that age has to be taken into account in evaluating the first-generation trials, because if you are comparing chemotherapy to nothing in young patients, you are seeing not only cytotoxic effects but also the effects due to ovarian ablation or suppression.
Young patients with ER-positive disease may be a little less responsive to the cytotoxic effects of chemotherapy, but the adjuvant endocrine effects they are also receiving brings up their response. Overall, ER status did not appear to make a difference in these patients.
DR RAVDIN: This was hotly debated and complicated by the fact that age has to be taken into account in evaluating the first-generation trials, because if you are comparing chemotherapy to nothing in young patients, you are seeing not only cytotoxic effects but also the effects due to ovarian ablation or suppression.
Young patients with ER-positive disease may be a little less responsive to the cytotoxic effects of chemotherapy, but the adjuvant endocrine effects they are also receiving brings up their response. Overall, ER status did not appear to make a difference in these patients.
That being said, this analysis describes the benefit of chemotherapy versus nothing. The regimens that included taxanes were never compared to nothing.They were compared to regimens that had already resulted in ovarian ablation in many patients who would have experienced it. In those patients, the effects of the taxanes appeared equal between older and younger women, irrespective of ER status. That was a surprise because I would have expected patients with ER-negative disease to be more chemosensitive than those with ER-positive disease. In fact, the taxanes appeared to be somewhat more effective with ER-positive disease, irrespective of age or ER status.
![]() DR LOVE: Would it be fair to conclude that this finding contradicts the analysis by Berry and colleagues (Berry 2006)?
DR LOVE: Would it be fair to conclude that this finding contradicts the analysis by Berry and colleagues (Berry 2006)?
![]() DR RAVDIN: In a way it does. I’ve heard people say, “Patients with ER-positive disease don’t benefit from chemotherapy, particularly if they’re older, and these patients shouldn’t be treated with chemotherapy.” I believe this is an inaccuracy and an oversimplification.
DR RAVDIN: In a way it does. I’ve heard people say, “Patients with ER-positive disease don’t benefit from chemotherapy, particularly if they’re older, and these patients shouldn’t be treated with chemotherapy.” I believe this is an inaccuracy and an oversimplification.
Patients with ER-positive disease benefit, although they benefit less than those with ER-negative tumors. In fact, with first-generation regimens, patients with ER-positive disease benefit dramatically less than those with ER-negative disease, but with second-generation regimens, the difference begins to be obscured by the addition of agents that are effective in both patient groups.
![]() DR LOVE: Were there any data presented on the time course of recurrence in ER-positive versus ER-negative disease?
DR LOVE: Were there any data presented on the time course of recurrence in ER-positive versus ER-negative disease?
![]() DR RAVDIN: In the first-generation trials, chemotherapy was equally beneficial
in ER-positive and ER-negative disease during the early years, when chemotherapy is most effective against recurrence. The same was observed in the middle time period. During the late time period, the data start to become noisy, and the effectiveness of those regimens against late relapse seemed to decrease to about the same level in all patients.
DR RAVDIN: In the first-generation trials, chemotherapy was equally beneficial
in ER-positive and ER-negative disease during the early years, when chemotherapy is most effective against recurrence. The same was observed in the middle time period. During the late time period, the data start to become noisy, and the effectiveness of those regimens against late relapse seemed to decrease to about the same level in all patients.
One could argue, “For those first-generation regimens, patients with ER-positive and ER-negative disease should have benefited equally.” But that’s not true. Patients with ER-negative disease tend to experience early relapses, when therapy is most effective against relapses. In contrast, ER-positive disease tends to have later relapses, say after three years. Therefore, when you look cumulatively at the impact of therapy after 10 years, you see a difference.
We already see there are some important differences between ER-negative and ER-positive patients. What we haven’t really seen before is that the effectiveness in given time intervals between ER-positive and ER-negative looks rather similar, which is a surprise to me. It emphasizes that the time course of recurrence is important and that it differs between ER-positive and ER-negative disease. In looking at early versus late relapse, I believe we will gain some interesting insights.
![]() DR LOVE: Is there a rule for calculating the late risk of relapse? I know you have that in your Adjuvant! Online computer program.
DR LOVE: Is there a rule for calculating the late risk of relapse? I know you have that in your Adjuvant! Online computer program.
![]() DR RAVDIN: Adjuvant! (adjuvantonline.com) considers the time course of relapse for patients with node-positive versus node-negative disease and whether to give hormonal therapy (Ravdin 2001).
DR RAVDIN: Adjuvant! (adjuvantonline.com) considers the time course of relapse for patients with node-positive versus node-negative disease and whether to give hormonal therapy (Ravdin 2001).
Late relapse rates are higher among patients with node-positive than node-negative disease, but they are not as dramatically different as they are during the early period. On average, the late relapse rate was about four percent per year for node-positive and two percent per year for node-negative disease.
Track 5
![]() DR LOVE: What else was presented at the Oxford meeting that you believe is important for clinicians to know?
DR LOVE: What else was presented at the Oxford meeting that you believe is important for clinicians to know?
![]() DR RAVDIN: With regard to hormonal therapy, we are all looking forward to data from the big trials of five versus 10 years of tamoxifen. The ATLAS (Adjuvant Tamoxifen — Longer Against Shorter) and ATTOM (Adjuvant Tamoxifen Treatment Offers More) trials are still blinded and ongoing. As we’ve seen with endocrine therapies and aromatase inhibitors, trials with particularly positive results or survival benefits are stopped early. I believe we can infer that the ATLAS and ATTOM trials are not strikingly positive, which is reassuring to American oncologists.
DR RAVDIN: With regard to hormonal therapy, we are all looking forward to data from the big trials of five versus 10 years of tamoxifen. The ATLAS (Adjuvant Tamoxifen — Longer Against Shorter) and ATTOM (Adjuvant Tamoxifen Treatment Offers More) trials are still blinded and ongoing. As we’ve seen with endocrine therapies and aromatase inhibitors, trials with particularly positive results or survival benefits are stopped early. I believe we can infer that the ATLAS and ATTOM trials are not strikingly positive, which is reassuring to American oncologists.
![]() DR LOVE: What about the aromatase inhibitors?
DR LOVE: What about the aromatase inhibitors?
![]() DR RAVDIN: The aromatase inhibitors are interesting. Looking at all the trials, including the “switching” studies, the proportional benefit for an aromatase inhibitor over tamoxifen was about 20 percent for recurrence and 10 percent for survival.
DR RAVDIN: The aromatase inhibitors are interesting. Looking at all the trials, including the “switching” studies, the proportional benefit for an aromatase inhibitor over tamoxifen was about 20 percent for recurrence and 10 percent for survival.
The aromatase inhibitor trials demonstrate the limitations of an overview analysis. To statisticians, all aromatase inhibitor trials look alike, whereas to clinicians, all aromatase inhibitor trials look different. A number of us, including myself, had a crisis of confidence in the overview process knowing that all of these trials would be lumped together.
It’s not necessarily a good thing to apply the overview analysis to a group of trials in a general sense. In fact, it can obscure important differences.
Moreover, I don’t believe that a meta-analysis can address the question that a lot of us want answered: Should I be using an aromatase inhibitor up front, or should I be administering two or three years of tamoxifen and then using an aromatase inhibitor?
Track 7
![]() DR LOVE: Where are you in terms of integrating trastuzumab data into the Adjuvant! Online program?
DR LOVE: Where are you in terms of integrating trastuzumab data into the Adjuvant! Online program?
![]() DR RAVDIN: Now that the trastuzumab trials have been formally published, we are able to evaluate and include the data. Currently, the program doesn’t make projections for trastuzumab outcomes at 10 years because we have data with follow-up of only two to three years.
DR RAVDIN: Now that the trastuzumab trials have been formally published, we are able to evaluate and include the data. Currently, the program doesn’t make projections for trastuzumab outcomes at 10 years because we have data with follow-up of only two to three years.
Many of the patients with ER-positive disease will experience recurrence later. If we don’t know that part of the story, we could give wildly inaccurate estimates.
Version 9 of the breast cancer program is about to be released. For the first time, it includes HER2 status as one of the program parameters. The 9.0 program provides a separate output for trastuzumab, projecting benefit at five years, which is reasonable to talk about. Some patients have been followed for five years in the trastuzumab trials. The program also provides information about some of the toxicities and uncertainties about toxicity.
![]() DR LOVE: Understanding the caveat of not having the longer follow-up that the other modalities have, what’s the number you’re going to put in there in terms of relative reduction of recurrence and mortality for trastuzumab?
DR LOVE: Understanding the caveat of not having the longer follow-up that the other modalities have, what’s the number you’re going to put in there in terms of relative reduction of recurrence and mortality for trastuzumab?
![]() DR RAVDIN: The literature-based estimates are about one third for mortality and 50 percent for recurrence. Those are the numbers that the program will use.
DR RAVDIN: The literature-based estimates are about one third for mortality and 50 percent for recurrence. Those are the numbers that the program will use.
![]() DR LOVE: HER2 will be a completely separate variable? ER status, et cetera, won’t matter, and trastuzumab will cut the recurrence rate in half?
DR LOVE: HER2 will be a completely separate variable? ER status, et cetera, won’t matter, and trastuzumab will cut the recurrence rate in half?
![]() DR RAVDIN: Correct, but remember that all the trials thus far have not evaluated
the one question that a lot of us would like to know: What would be the impact of trastuzumab alone? A lot of people may not be enthusiastic about chemotherapy, but the limitation of the data limits the program.
DR RAVDIN: Correct, but remember that all the trials thus far have not evaluated
the one question that a lot of us would like to know: What would be the impact of trastuzumab alone? A lot of people may not be enthusiastic about chemotherapy, but the limitation of the data limits the program.
We don’t have any results on the impact of trastuzumab alone. We only have randomized trial results of the impact of trastuzumab when added to adjuvant chemotherapy.