|
You are here: Home: BCU 3|2001: Section 2
Section 2
Is
Four Cycles of AC Adequate Adjuvant Chemotherapy?
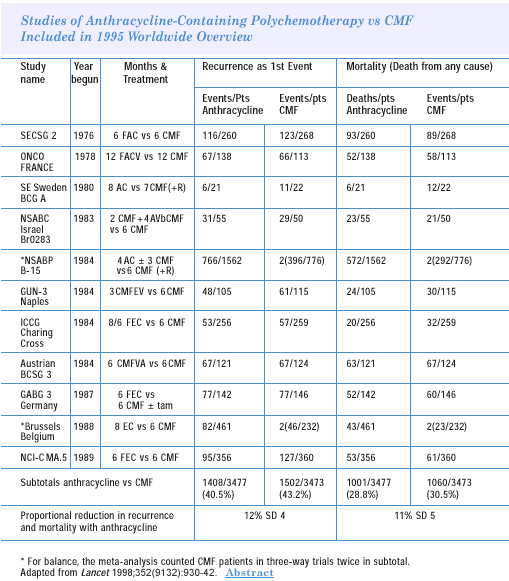
The 1995 overview established that anthracycline-containing
regimens added benefit, but the choice of a specific regimen is
also an issue. My reading of the literature is that we were too
hasty in adopting four cycles of AC as a standard, and it was based
more on convenience than true superiority over the previous standard,
which was CMF. In fact, there are no convincing data that AC times
four is superior to CMF.
On the other hand, if you look at the overview of randomized trials,
with and without anthracyclines, there is superiority in favor of
the anthracyclines. Most of that comes from three-drug regimens.
And you can interpret that as either the contribution of 5-fluorouracil
— which is usually the third drug — or the contribution
of duration of therapy. Most of the three-drug combinations have
been administered for six cycles, as opposed to the AC or EC regimens,
which were given mostly for four cycles. In the adjuvant setting,
one should try to use what has — at least on the basis of controlled
trials — given the best results. In this case, I think we have
compromised instead of going for the best.
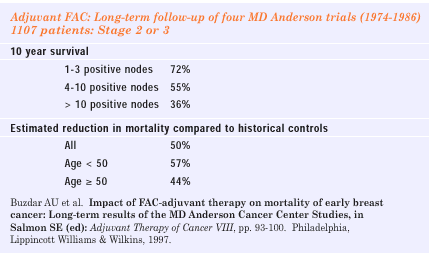
I do not have
incontrovertible proof that my hypothesis is completely correct,
but I think there is more evidence on this side than on the other.
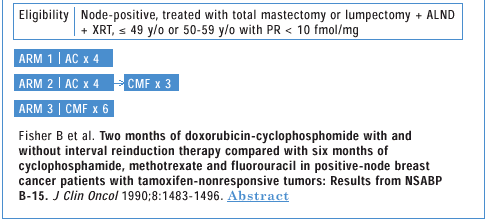
And if you read the original NSABP B-15 paper that compared AC versus
CMF, it is clear that the reason for recommending adoption of AC
is simply one of convenience. I have stated this in public, including
at the NIH Consensus Development Conference, although I was unsuccessful
in making this sufficiently clear to the panel to sway their opinion.
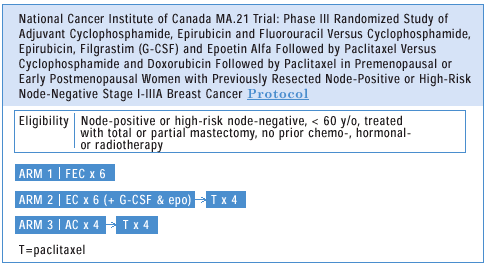
The Canadians are pursuing a clinical trial that will go to the
heart of this matter by comparing FEC versus EC/Taxol versus AC/Taxol.
—
Gabriel Hortobagyi, MD
|
NIN
CONSENSUS DEVELOPMENT CONFERENCE ON EARLY BREAST CANCER, FINAL
STATEMENT FULL
TEXT
…Available
data indicate that adjuvant chemotherapy regimens that include
an anthracycline result in a small but statistically significant
improvement in survival compared to nonanthracycline-containing
programs.
…Randomized trials have demonstrated threshold dose effects
for two of the most active chemotherapeutic agents, doxorubicin
(A) and cyclophosphamide (C). These two drugs are frequently
administered together (AC) and appear to result in a comparable
survival outcome, whether given preoperatively or postoperatively.
However, AC has not been compared to cyclophosphamide/doxorubicin/5-fluorouracil
(CAF) or cyclophosphamide/epirubicin/5-fluorouracil (CEF).
|


SELECT
PUBLICATIONS
|
Polychemotherapy
for early breast cancer: An overview of the randomised trials.
Early Breast Cancer Trialists’ Collaborative Group. Lancet
1998;352(9132):930-42. Abstract
Bang SM et al. Adjuvant doxorubicin and cyclophosphamide
versus cyclophosphamide, methotrexate and 5-fluorouracil chemotherapy
in premenopausal women with axillary lymph node-positive breast
carcinoma. Cancer 2000;89(12):2521-6. Abstract
Buzzoni R et al. Adjuvant chemotherapy with doxorubicin
plus cyclophosphamide, methotrexate and fluorouracil in the
treatment of resectable breast cancer with more than three
positive axillary nodes. J Clin Oncol 1991;9(12):2134-40.
Abstract
Coombes RC et al. Adjuvant cyclophosphamide,methotrexate
and fluorouracil versus fluorouracil,epirubicin and cyclophosphamide
chemotherapy in premenopausal women with axillary node-positive
operable breast cancer: Results of a randomized trial.The
International Collaborative Cancer Group. J Clin Oncol
1996;14(1):35-45. Abstract
Fisher B et al. Two months of doxorubicin-cyclophosphamide
with and without interval reinduction therapy compared with
six months of cyclophosphamide, methotrexate and fluorouracil
in positive-node breast cancer patients with tamoxifen-nonresponsive
tumors:Results from NSABP B-15. J Clin Oncol 1990;
8:1483-1496. Abstract
Hortobagyi
GN. Overview:Progress in systemic chemotherapy of primary
breast cancer. NIH Consensus Conference on Early Breast
Cancer 2000. Abstract
Ibrahim NK et al. Doxorubicin-based adjuvant chemotherapy
in elderly breast cancer patients:The MD Anderson experience
with long-term follow-up. Ann Oncol 2000;11(12):1597-601.
Abstract
Kaufmann M et al. Adjuvant randomized trials of doxorubicin/
cyclophosphamide versus doxorubicin/cyclophosphamide/ tamoxifen
and CMF chemotherapy versus tamoxifen in women with node-positive
breast cancer. J Clin Oncol 1993;11(3):454-60.
Abstract
Levine MN et al. Randomized trial of intensive clophosphamide,
epirubicin and fluorouracil chemotherapy compared with cyclophosphamide,
methotrexate and fluorouracil in premenopausal women with
node-positive breast cancer. National Cancer Institute of
Canada Clinical Trials Group. J Clin Oncol 1998;16(8):2651-8.
Abstract
Wood WC et al. Dose and dose intensity of adjuvant chemotherapy
for stage II, node-positive breast carcinoma. N
Engl J Med 1994;330 (18):1253-9. Abstract
|
|
|
