|
You are here: Home: BCU 3|2001: Section 5
Section 5
Combination Endocrine Therapy
LHRH
AGONISTS PLUS TAMOXIFEN
Combination
endocrine therapy is a conceptual change for us. We eliminated that
approach a couple of decades ago,perhaps because of small, inadequately
powered trials that were clearly unable to detect the type of differences
we can identify today with larger studies. The reality is that combination
endocrine therapy may be more effective than single agent treatment.
With better tools now available, we are returning to the concept
of complete estrogen blockade — a strategy that started several
decades ago with hypophesectomy and adrenalectomy. Studies of chemical
ovarian suppression plus tamoxifen suggest greater efficacy than
either alone.
—Gabriel
Hortobagyi, MD
The combination
of an LHRH agonist and tamoxifen would be interesting to study in
premenopausal women with large tumors that are strongly ER-positive.
We found that Zoladex plus tamoxifen in the neoadjuvant setting
produce better and longer-lasting responses than either agent alone
or in sequence. So, it ’s something that deserves further exploration.
We are also planning to study the mechanism of action of Faslodex
® (fulvestrant) using microarray and also combining it with
other agents. The combination of Faslodex and Arimidex looks very
interesting because of the different mechanisms of action.
—J
Michael Dixon, MD
ARIMIDEX
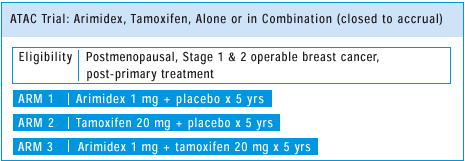
PLUS TAMOXIFEN OF THE ATAC TRIAL: ANTITUMOR EFFECTS
On the basis
of emerging data in premenopausal women with chemical ovarian ablation
and tamoxifen, one would expect that the combination arm of ATAC
would be superior to the other two arms. On the other hand, there
is some preclinical data suggesting that the combination of tamoxifen
and anastrozole will not be advantageous, so we ’ll have to
wait for the data. In terms of the single agent ATAC arms, I am
impressed by the data from the large aromatase inhibitor trials
in first-line metastatic breast cancer, and on that basis, we have
the hypothesis that the same type of comparison in the adjuvant
setting will have a similar or magnified outcome.
—Gabriel
Hortobagyi, MD
From the data
in the metastatic and neoadjuvant setting, it’s quite clear
that aromatase inhibitors are superior to tamoxifen. I would expect
that superiority to carry through into the adjuvant setting, although
it may take some time to observe.The use of aromatase inhibitors
in the adjuvant setting is not currently approved, but for high-risk,
ER-positive, postmenopausal patients with a previous stroke or DVT,
we consider substituting an aromatase inhibitor.
The combination
arm of ATAC is more difficult to predict, but the in vivo effects
of Arimidex and tamoxifen are very different — they work through
different mechanisms — and this is the major background basis
to believe that the combination arm might be better.
—
J Michael Dixon, MD
ARIMIDEX
PLUS TAMOXIFEN ARM OF ATAC TRIAL: BONE, LIPID, ENDOMETRIAL EFFECTS
I expect some
impact of Arimidex on the bones, but probably not a major effect.
Many of our neoadjuvant patients have taken anastrozole or letrozole
for five years, and we have not seen major problems as far as side
effects are concerned. One reason that the UK prevention study decided
not to have a combination arm is that there ’s not enough information
yet on the safety profile of combining Arimidex and tamoxifen. Everybody’s
waiting with baited breath to see the results of the ATAC study.
In the combination arm, there are competing effects of Arimidex
lowering estrogen levels and
tamoxifen’s estrogen-mimicking action on bone and lipids.The
hope is that any adverse effects of Arimidex will be countered by
the positive effects of tamoxifen. So, from a theoretical point
of view, you ’d expect either no effect or perhaps even a positive
effect. In terms of the endometrium, theoretically the combination
should be no worse than tamoxifen alone. Arimidex causes less vaginal
problems than tamoxifen and no leukorrhea.
—
J Michael Dixon, MD
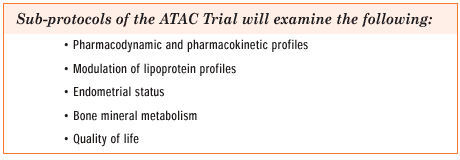
The ATAC trial
has subprotocols looking at bone,lipids and the endometrium that
are appropriately powered to answer those important questions. However,
in the first-line trials of aromatase inhibitors, there was no increase
in bone-related complications such as compression fractures. I would
expect a higher fraction of patients to remain disease-free in the
anastrozole arm, and the main question is the combined arm —
where you may have greater antitumor activity plus some of the benefits
of Tamoxifen, for example, on bone. We don’t have many patients
in the metastatic setting exposed to aromatase inhibitors for extended
periods of time. If ATAC looks positive, I would have serious reservations
about substituting another aromatase inhibitor for long-term adjuvant
therapy, particularly because of safety concerns.
—
Aman Buzdar, MD
AROMATASE
INHIBITORS IN WOMEN MADE MENOPAUSAL BY LHRH AGONISTS
There is one
limited metastatic study in ER-positive patients demonstrating that
Zoladex plus anastrozole yielded similar responses to what has been
seen in postmenopausal patients, but we need to design appropriate
trials to address this issue.It is very important to consider that
aromatase inhibitors as monotherapy should not be used in premenopausal
patients. But it would be a very interesting and logical approach
to design trials of complete estrogen blockade in ER-positive premenopausal
patients, using an LHRH agonist and anastrozole.
—Aman
Buzdar, MD
SELECT
PUBLICATIONS
|
Baum M
et al. Management of premenopausal women with early breast
cancer: Is there a role for goserelin? Proc ASCO
2001; Abstract
103.
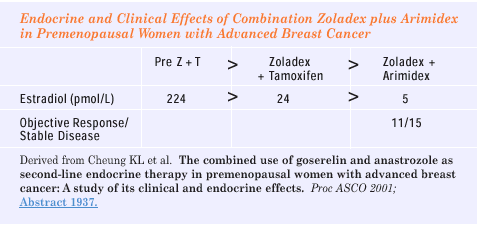
Cheung
KL et al. The combined use of goserelin and anastrozole
as second-line endocrine therapy in premenopausal women with
advanced breast cancer: A study of its clinical and endocrine
effects. Proc ASCO 2001; Abstract
1937.
Dowsett
M. Drug and hormone interactions of aromatase inhibitors.
Endocr Rel Ca 1999;6(2):181-185. Full
Text
Ingle
JN et al. Combination hormonal therapy involving aromatase
inhibitors in the management of women with breast cancer.
Endocr Relat Cancer 1999;6:265-9. Full
Text
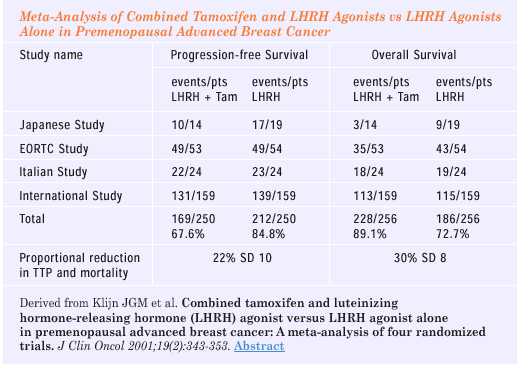
Klijn JG et al. Combined tamoxifen and luteinizing hormone-releasing
hormone (LHRH) agonist versus LHRH agonist alone in premenopausal
advanced breast cancer: A meta-analysis of four randomized
trials. J Clin Oncol 2001;19:343-53. Abstract
Michaud LB, Buzdar AU. Complete estrogen blockade for the
treatment of metastatic and early stage breast cancer.
Drugs Aging 2000;16:261-71. Abstract
Nystedt
M et al. Randomized trial of adjuvant tamoxifen and/or
goserelin in premenopausal breast cancer — self-rated
physiological effects and symptoms. Acta Oncol 2000;39(8):959-68.
Abstract
|
|
|
