|
You are here: Home: BCU 3|2001: Section 6
Section
6
Tamoxifen and Quality of Life
In the NSABP
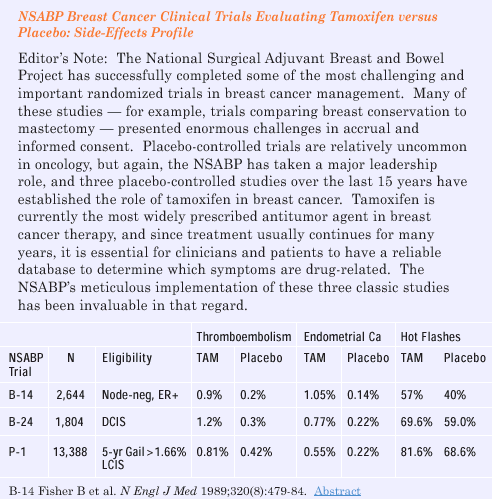
P-1 prevention trial, hot flashes, sweats and vaginal discharge
were the only symptoms found in excess in women taking tamoxifen
compared to placebo. But women who were less than age 50 when they
started tamoxifen did not have a substantial increase in either
hot flashes or sweats, and in my clinical experience, hot flashes
and sweats are relatively infrequent in breast cancer patients who
are still well-estrogenized from circulating ovarian hormones. Vaginal
discharge also does not seem to be increased in younger women. Overall,
women less than age 50 have the best risk-benefit profile both in
terms of day-to-day toxicity and/or serious adverse events.
In the prevention
trial, we used very well-validated and established measures of depression,
sexual functioning and quality of life. On all of these, we saw
absolutely no difference between the tamoxifen and placebo groups.
There was no effect that we could measure on physical functioning,
emotional well-being, fatigue, social interaction or pain. And because
the sample size was so huge — we evaluated about 11,000 women
— even a one percent difference could be detected, and there
was no difference in quality of life over time.
What this says
to me is that while there are symptoms that are somewhat annoying,
they don’t affect these other aspects of life. And, again,
importantly, we didn’t see any excess depression, something
that had been previously attributed to tamoxifen.
Another issue
was weight gain, which is extremely common in women as they age.
This a phenomenon of the menopausal transition, because the metabolic
rate declines as women go through menopause. In the prevention trial
— at least by self-report — there was no difference in
body image or in reports of weight gain in patients on tamoxifen
versus placebo.
—Patricia
Ganz, MD

SELECT
PUBLICATIONS
|
Berglund
G et al. Effect of endocrine treatment on sexuality in
premenopausal breast cancer patients: A prospective randomized
study. J Clin Oncol 2001;2788- 2796. Abstract
Breuer B, Anderson R. The relationship of tamoxifen with
dementia,depression and dependence in activities of daily
living in elderly nursing home residents. Women Health
2000;31:71-85. Abstract
Cushman
M et al. Tamoxifen and cardiac risk factors in healthy
women: Suggestion of an anti-inflammatory effect. Arterioscler
Thromb Vasc Biol 2001;21:255-61. Abstract
Day R
et al. Health-related quality of life and tamoxifen in
breast cancer prevention: A report from the National Surgical
Adjuvant Breast and Bowel Project P-1 study. J Clin
Oncol 1999:2659. Abstract
Demissie
S et al. Adjuvant tamoxifen:Predictors of use, side effects
and discontinuation in older women. J Clin Oncol 2001;19:322-8.
Abstract
Fallowfield
L et al. Tamoxifen for the prevention of breast cancer:
Psychosocial impact on women participating in two randomized
controlled trials. J Clin Oncol 2001;19:1885-92.
Abstract
Fisher
B et al. A randomized clinical trial evaluating tamoxifen
in the treatment of patients with node-negative breast cancer
who have estrogen receptor-positive tumors. N Engl
J Med 1989;320(8):479-84. Abstract
Fisher
B et al. Tamoxifen in treatment of intraductal breast cancer:
National Surgical Adjuvant Breast and Bowel Project B-24 randomised
controlled trial. Lancet 1999;353(9169):1993-2000.
Abstract
Fisher
B et al. Tamoxifen for prevention of breast cancer: Report
of the National Surgical Adjuvant Breast and Bowel Project
P-1 Study. J Natl Cancer Inst 1998;90(18):1371-88.
Abstract
Ganz PA
et al. Life after breast cancer: Understanding women ’s
health-related quality of life and sexual functioning.
J Clin Oncol 1998;16(2):501-14. Abstract
Ganz PA et al. Impact of different adjuvant therapy strategies
on quality of life in breast cancer survivors. Recent
Results Cancer Res 1998;152:396-411. Abstract
Goodwin
PJ et al. Risk of menopause during the first year after
breast cancer diagnosis. J Clin Oncol 1999;17(8):2365-70.
Abstract
Mourits
MJ et al. Tamoxifen treatment and gynecologic side effects:
A review. Obstet Gynecol 2001;97(5 Pt 2):855-66.
Abstract
|
OTHER
RESOURCES
Questions and
Answers about Tamoxifen: An NCI Publication for Patients
Full
Text
|
|
