|
You are here: Home: BCU 3|2001: Section 9
Section 9
Menopause and Hormone Replacement
in Breast Cancer Patients
MENSTRUAL
CYCLE AND QUALITY OF LIFE: A PROSPECTIVE STUDY CURRENTLY
RECRUITING PATIENTS
Editor’s
Note: On the accompany audio program, Dr Jeanne Petrek reviews
the current available data base on the effects of breast
cancer therapies on reproductive function. To enhance the
understanding of this important therapeutic issue, Dr Petrek
is recruiting premenopausal breast cancer patients to participate
in a prospective study that consists of regularly mailed
surveys for patients to complete.
Eligibility:
Women with invasive breast cancer, age 18-45, with regular
menstrual cycles, within six months of diagnosis
Objectives
of the study are to identify:
Protocol:
Chart review, patient interview and data collection through
questionnaires mailed directly to and to be completed by
the subjects themselves. Note that this multicenter study
has received IRB approval. It is not necessary for physicians
to obtain local individual IRB approval to participate.
Interested patients may enroll themselves.
Contact information: Lisa Loudon, Memorial Sloan-Kettering
Cancer Center
Phone: 1-877-636-7562 Email: ruddl@mskcc.org
|
CHEMOTHERAPY
AND PREMATURE MENOPAUSE
For women approaching
the age for natural menopause, even relatively small doses of alkylating
agents can cause permanent amenorrhea. However, younger women can
receive very intensive regimens and not even miss a menstrual period.
So age is a major determinant.
At Memorial
Sloan-Kettering, very early stage patients are often given the choice
of traditional CMF for six months versus four doses of AC, and with
the lesser amount of alkylating agent in the AC, the patient should
have a better chance of preserving fertility. So a woman can choose
a regimen that is less likely to cause hair loss (CMF) or one that
is less likely to result in premature menopause (AC).
—
Jeanne Petrek, MD
HORMONE
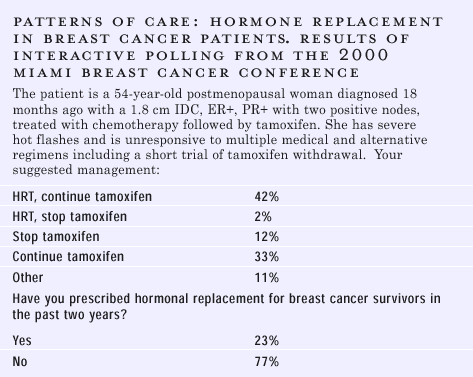
REPLACEMENT THERAPY IN THE BREAST CANCER PATIENTS
Most of the
data on HRT in breast cancer patients come from case series, and
those that have been published do not show an excess risk of recurrence.
But, that’s not as clean as randomized controlled trials. The
key issue is risk for a new breast cancer. If it’s short term,
then a year or two of HRT is probably not going to make a big difference
in terms of risk of a new breast cancer — for example, a 40-year-old
woman who has been made prematurely menopausal from chemotherapy
and is overwhelmed by hot flashes. On the other hand, if the patient
has a high risk for metastases, some physicians might feel very
uncomfortable giving HRT. I just don’t think we have the data.
Hormone replacement
therapy is clearly very beneficial for management of hot flashes
and sweats, and for some women who can’t get relief with alternative
therapies, HRT may be the only way to get these symptoms under control.
So I do treat breast cancer patients with HRT if they need it. Vaginal
dryness is also a major symptom, and there is now available a topical
therapy — Estring® (estradiol vaginal ring), a silastic
impregnated ring that can be placed in the back of the vagina and
left there for three months, slowly releasing estrogen. This provides
great benefit for patients, and there’s no systemic absorption
of estrogens.
Our experience
with very symptomatic menopausal breast cancer patients is that
they are risk-averse and often don’t want to take anything.
There is this kind of “grin-and-bear-it” attitude. With
that in mind, when a woman is down to the point where she wants
to be treated, we shouldn ’t be discouraging her from obtaining
relief of symptoms, which are probably going to be short-lived.
I have been able to give permission to women to do this, and I think
we have to be able to wear that other hat and not be so risk-averse.
There are liability issues and all sorts of other things, but in
a woman who is not sleeping, having very poor quality of life and
cannot function at work, why should she be denied something that
is effective?
—
Patricia Ganz, MD
EFFECTS
OF HRT IN NON-BREAST CANCER PATIENTS
There were some
excess cardiovascular deaths early on in the Women’s Health
Initiative, so we can’t be confident at this point that there’s
going to be a long-term benefit. The issue of cognitive functioning
is another question mark. We don’t have good randomized trial
data and, again, the Women’s Health Initiative hopefully will
help with that. So I ask patients, “What is the reason for
considering HRT? If you ’re really symptomatic, take it, because
that’s the best way to manage symptoms.” Also, women who
are going through menopause tend to have vasomotor symptoms for
a two-or three-year period and then it tends to trail off.
There was a
meta-analysis of HRT studies, and overall, there was about a 35
percent increased risk of breast cancer. However, this meta-analysis
was primarily from women who had been on estrogen alone. Two recent
articles suggested that it was the progestational component that
was the major culprit in terms of an increased risk, although we
can’t be clear about that. The data do suggest that the risk
increases primarily during therapy, and if a woman takes HRT for
a short period of time for symptoms and decides to stop, she’s
not going to have a long-term residual risk.
—
Patricia Ganz, MD
SELECT
PUBLICATIONS
|
Bieber
EJ,Barnes RB. Breast cancer and HRT — What are the
data? Int J Fertil Womens Med 2001;46:73-8. Abstract
Col NF et al. Hormone replacement therapy after breast
cancer: A systematic review and quantitative assessment of
risk. J Clin Oncol 2001;19:2357-63. Abstract
El-Bastawissi AY et al. Reproductive and hormonal factors
associated with mammographic breast density by age (United
States). Cancer Causes Control 2000;11:955-63.
Abstract
Ganz PA
et al. Managing menopausal symptoms in breast cancer survivors:
Results of a randomized controlled trial. J Natl Cancer
Inst 2000;92(13):1054-64. Abstract
Gapstur
SM et al. Hormone replacement therapy and risk of breast
cancer with a favorable histology: Results of the Iowa Women
’s Health Study. JAMA 1999;281(22):2091-7.
Abstract
Jacobs
HS. Hormone replacement therapy and breast cancer.
Endocrine-Related Cancer 2000;7(1):53-61. Full
Text
Manjer
J et al. Increased incidence of small and well-differentiated
breast tumours in postmenopausal women following hormone-replacement
therapy. Int J Cancer 2001;92:919-22. Abstract
Marsden
J et al. Are randomized trials of hormone replacement therapy
in symptomatic women with breast cancer feasible? Fertil
Steril 2000;73:292-9. Abstract
Nerhood
RC. Making a decision about ERT/HRT. Evidence to consider
in initiating and continuing protective therapy. Postgrad
Med 2001;109:167-70,173-4, 178. Full
Text
O ’Meara
ES et al. Hormone replacement therapy after a diagnosis
of breast cancer in relation to recurrence and mortality.
J Natl Cancer Inst 2001;93:754-61. Abstract
Pritchard
KI. The role of hormone replacement therapy in women with
a previous diagnosis of breast cancer and a review of possible
alternatives. Ann Oncol 2001;12:301-10. Abstract
Ross RK
et al. Effect of hormone replacement therapy on breast
cancer risk: Estrogen versus estrogen plus progestin.
J Natl Cancer Inst 2000;92:328-32. Abstract
Rutter CM et al. Changes in breast density associated with
initiation,
discontinuation and continuing use of hormone replacement
therapy. JAMA 2001;285:171-6. Abstract
Sellers
TA et al. The role of hormone replacement therapy in the
risk for breast cancer and total mortality in women with a
family history of breast cancer. Ann Intern Med 1997;127(11):973-80.
Abstract
Torgerson
DJ, Bell-Syer SEM. Hormone replacement therapy and
prevention of nonvertebral fractures: A meta-analysis of randomized
trials. JAMA 2001;285:2891-2897. Abstract
|
|
|
