| You are here: Home: BCU 3|2002: Harold Burstein, MD
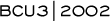

Edited comments by Dr Burstein
neoadjuvant trastuzumab/paclitaxel trial
Given that the patient population had large HER2-positive tumors, which have historically been more refractory to therapy — I’m very encouraged. This study is novel for several reasons. It is the first trial evaluating neoadjuvant trastuzumab, and there is a lot of interest in defining the response rate. Also, we performed cardiac analyses during the trastuzumab/paclitaxel therapy and again during the postsurgical adjuvant AC chemotherapy. Our results are very similar to George Sledge’s — a significant number of women had a 10-20% decline in their ejection fraction. Fortunately, none of the patients developed any symptoms of congestive heart failure, and the changes in ejection fraction appear to reverse with time.
The decline in ejection fraction occurred either during or at the end of adjuvant AC in three of the four women, and did not change much during the trastuzumab/paclitaxel therapy. Most of us believe these kinds of changes in ejection fraction are consistent with what occurs with AC alone, but since this is not a randomized trial, we do not know if the addition of trastuzumab influences the ejection fraction.
influence of trastuzumab on her2 expression
Our study is the first to evaluate tumor samples before and after trastuzumab therapy. We are in the process of analyzing these results. It will be very interesting to determine the influence of trastuzumab exposure on a tumor’s HER2 status. There are confounding factors, however, because some women had no residual tumor to test after trastuzumab therapy. Of course, those are the women in whom one would be most curious as to what was going on.
As a first-order analysis, it appears to be technically feasible to test for HER2 status after exposure to trastuzumab. Grossly, the tumors appear as if there is some treatment effect. They do not look particularly different from tumors treated with chemotherapy alone. Beyond that, we’re still evaluating the data.
Another component of the study is tracking the serologic response to neoadjuvant trastuzumab by measuring the circulating extracellular domain (ECD) of the HER2 protein. In women who are responding, the serum HER2 ECD is a good tumor marker, and women who respond tend to have a decline in their serum HER2 ECD.
timing of trastuzumab: implications for neoadjuvant and adjuvant therapy
A larger randomized trial evaluating neoadjuvant trastuzumabbased therapy would be very interesting. For chemotherapy, we have very convincingly shown that the sequence of treatment does not matter. There are several studies demonstrating that neoadjuvant chemotherapy is equivalent to adjuvant chemotherapy.
However in the metastatic setting, earlier trastuzumab exposure may be better than later trastuzumab exposure. This is based on the fact that two-thirds of the women in the trial by Slamon and colleagues, who received chemotherapy alone, subsequently received trastuzumab. Despite that crossover, there was still a survival advantage for those receiving chemotherapy plus trastuzumab initially compared to those receiving chemotherapy alone.
Most of the ongoing adjuvant trials with trastuzumab involve sequential chemotherapy first followed by chemotherapy plus trastuzumab as the control arm. But, I wonder if earlier exposure to trastuzumab might be clinically valuable.
adjuvant trastuzumab
We must be cautious when introducing therapies into the adjuvant setting. I do not use adjuvant trastuzumab outside of a clinical trial. In this situation, treatment as part of a clinical trial is better than treatment outside of a clinical trial, and there are adjuvant trials available at most large cancer centers.
Trastuzumab is a very promising drug, which has generated tremendous enthusiasm, but there are concerns about long-term side effects. While all of us hope to bring the answers to our patients as soon as possible, we have tried very hard to limit the use of adjuvant trastuzumab to patients on a study.
trastuzumab in combination with chemotherapy for metastatic disease
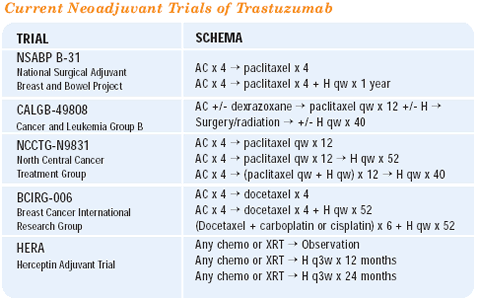
For a woman with HER2-positive, ER-negative breast cancer who has received prior AC and recently relapsed, trastuzumab in combination with chemotherapy is the standard. A variety of chemotherapeutic regimens have been evaluated in combination with trastuzumab — weekly paclitaxel, vinorelbine, docetaxel and docetaxel plus the platinums. These are all very effective regimens and very reasonable choices. We choose between these active regimens based upon their side-effect profiles and personal preferences. Very little data suggest that one regimen is superior to another. In women with neuropathies, one should be cautious about using drugs that cause neuropathy, and some women may not be willing to lose their hair, so you might choose regimens that are less likely to cause alopecia.
Our group has led the development of a randomized study, which we call the TRAVIOTA trial — trastuzumab and vinorelbine or a taxane. Women are randomized to either the trastuzumab/vinorelbine combination or to a trastuzumab/taxane combination. Physicians will be allowed to choose between weekly paclitaxel or weekly docetaxel. This is a 50-institution trial with an accrual goal of 250 patients. We want to objectively characterize the response rates and toxicity profiles for these regimens. I think we will find out that these are all very reasonable regimens and will have good news for patients in that there will be several options from which to choose.
duration of trastuzumab therapy
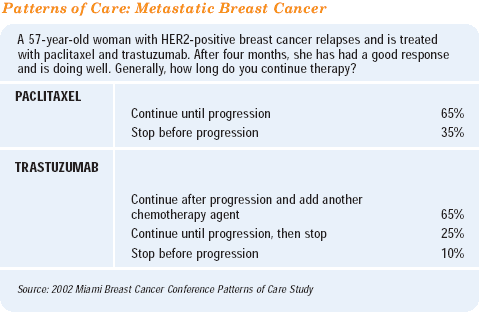
The duration of trastuzumab therapy is probably the most important question confronting us in the treatment of HER2- positive metastatic disease. We really do not have adequate data. The value of maintenance trastuzumab therapy is a huge question. In clinical practice, we often roll patients over from one trastuzumab-based chemotherapy regimen to another.
Initially, we treat women with trastuzumab plus chemotherapy. If they are fortunate to have a good response, we often stop the chemotherapy after 4-6 months and continue maintenance singleagent trastuzumab.
At the time of progression, there are a number of options. Most physicians would probably re-institute chemotherapy plus trastuzumab at that point. MD Anderson is conducting a randomized trial to assess the role of ongoing trastuzumab in such a setting. They are enrolling women who have progressed after receiving paclitaxel or docetaxel plus trastuzumab. These women are then randomized to either vinorelbine alone or vinorelbine plus trastuzumab.
treating women with her2-positive, er-positive disease
Some data suggest that women with HER2-positive, ER-positive disease may derive less benefit from hormone-based therapy than women with HER2-negative, ER-positive disease, but this does not mean they do not benefit.
Our clinical practice has been to use hormonal therapy as long as appropriate. When the woman needs chemotherapy, we introduce chemotherapy plus trastuzumab. Two sets of data support this practice. The pivotal trial with trastuzumab plus chemotherapy assessed the response rate as a function of ER status. Trastuzumab plus chemotherapy was equally effective in women with ER-positive and ER-negative disease. In women with ER-positive disease, the pivotal trial also evaluated the response rate as a function of prior hormonal therapy, which did not influence the response to trastuzumab plus chemotherapy.
Certainly, trastuzumab is active as a single agent, and women who do not want chemotherapy and are not candidates for further hormonal therapy could potentially start on single-agent trastuzumab — this is a reasonable option.
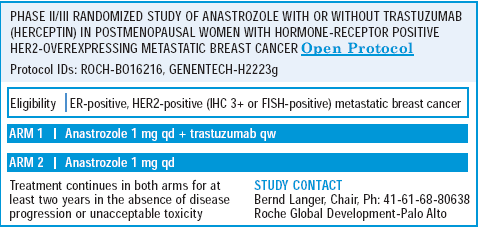
We are conducting a phase II trial evaluating an aromatase inhibitor in combination with trastuzumab. Without a randomized trial, we do not have data that this is superior. Of course, this commits the woman to a weekly treatment, and the beauty of hormonal therapy, aside from its effectiveness, is its convenience.
preliminary results of the atac trial
The ATAC trial is the largest single randomized trial — with more than 9,000 women — conducted in breast cancer research. There are, however, several caveats. First, there was no survival difference, since there were very few deaths related to breast cancer. Second, there is a limited follow-up of only 2.5 years.
However, I think the time for the introduction of aromatase inhibitors in early-stage disease is rapidly arising. Compelling studies in metastatic disease and the neoadjuvant setting demonstrate that aromatase inhibitors are at least as good as tamoxifen. And now, we have this large adjuvant trial showing that anastrozole may be better than tamoxifen.
In the preliminary data from the ATAC trial, patients on anastrozole had fewer menopausal symptoms and a reduced rate of endometrial cancer and thromboembolic events. There were somewhat greater osteoporotic events in the anastrozole arm. It’s not clear if this is a detrimental effect associated with the aromatase inhibitors or if it just represents the background level of osteoporotic fractures, with tamoxifen increasing the bone mineral density a little bit. This is an issue that we want some long-term data on.
For women already receiving tamoxifen, I would leave them on tamoxifen. It is a very safe and effective drug with decades of proven experience. For women finishing their course of tamoxifen, it would be interesting to study the effects of sequencing treatments such as tamoxifen followed by an aromatase inhibitor. I encourage physicians to think about enrolling women on those trials, which are ongoing at multiple sites.
Newly diagnosed women may wish to be very familiar with the ATAC results, and it is likely that we will see aromatase inhibitors used in the adjuvant setting very soon. We have already been using adjuvant aromatase inhibitors in women with a family history of uterine cancer or a personal history of uterine cancer or blood clots.
adjuvant trials of breast cancer patients with chemotherapy-induced menopause
Women who become menopausal as a result of chemotherapy tend to do better in the long term. The NIH consensus conference in November 2000 identified the role of ovarian ablation in premenopausal women as an important question in breast cancer research. The ATAC data forces a re-analysis of this issue, because the question of whether you should suppress the ovaries and then add an aromatase inhibitor is now crucial.
The Intergroup, in collaboration with the Europeans, is planning a large randomized trial for premenopausal women to evaluate ovarian ablation in women who continue menstruating after chemotherapy. The trial will compare tamoxifen alone versus ovarian ablation plus tamoxifen versus ovarian ablation plus an aromatase inhibitor.
evaluation of innovative, nontoxic agents: ppar gamma agonists
At Dana-Farber, we were interested in the paroxisome proliferator activated receptors gamma — PPAR gamma — an interesting intracellular signaling family. Data indicate that stimulating the PPAR gamma chain can cause tumor cell differentiation in a variety of tumor cell lines, including breast cancer. If breast cancer cell lines are exposed to PPAR gamma agonists, the cells slow their rate of growth. There are commercially available drugs with this PPAR gamma agonist activity that are used to treat diabetes, so there is tremendous safety experience with these agents.
We conducted a small phase II study in very heavily pretreated women with metastatic breast cancer. Unfortunately, it did not make a significant clinical impact on the course of their disease. Nonetheless, it was impressive that within 7-8 months we were able to enroll 22 women to this trial. There is a wellspring of good faith among our patients. If we have innovative ideas to evaluate nontoxic treatments, patients are willing to explore them with you.
concurrent paclitaxel and radiation therapy
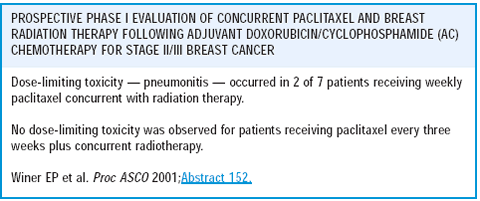
We have been interested in the concurrent use of chemotherapy and radiation therapy. There has been interest in adding a taxane to AC chemotherapy in the adjuvant setting, but that practice prolongs the length of therapy by an additional three months and typically places radiation therapy at the end. Our group was interested in determining if radiation therapy could be given at the same time as paclitaxel. We designed a phase I trial with patients receiving AC followed by paclitaxel with concurrent radiation, with paclitaxel administered either weekly or every three weeks.
Weekly paclitaxel with concurrent radiation was too toxic. Since paclitaxel is probably a radiosensitizer, weekly administration meant an unacceptably high risk of pulmonary complications, such as radiation-related pneumonitis. It did appear feasible to administer every-three-week paclitaxel with concurrent radiation therapy, but we need more data on this.
patient preferences for learning about the results of clinical trials
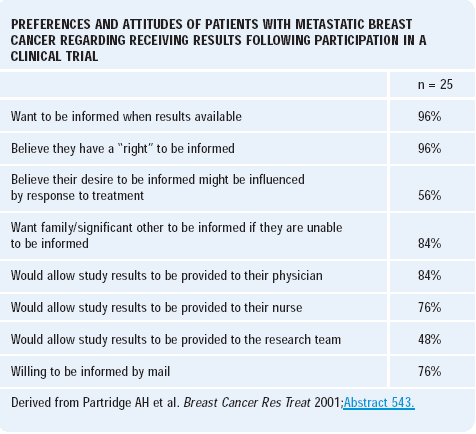
Ann Partridge at Dana-Farber conducted a study to evaluate patient preferences for learning about the results of clinical trials in which they participated. We do not usually share the clinical trial results with the participants in a systematic fashion, and no one has analyzed whether or not patients would like to learn this information.
The survey asked patients participating in a specific phase II clinical trial whether or not they would like to learn the results of the study. Of the patients responding to this survey, 96% indicated they were very interested in knowing the results. This particular phase II trial was not randomized, and patients’ opinions may differ for a randomized study.
It is a fascinating and very provocative study that really challenges the clinical research community to think about ways of communicating with patients what has been learned in clinical trials. Sharing that information in a respectful and appropriate manner is something that will be challenging.
Select publications
|
