| You are here: Home: BCU 3|2002: William Gradishar, MD

Edited comments by Dr Gradishar
capecitabine/docetaxel (xt) trial
Relatively few trials have demonstrated a survival benefit in women with metastatic breast cancer. The combination of capecitabine and docetaxel improved response rates and survival compared to singleagent docetaxel. Although there was a survival advantage, there was also somewhat greater toxicity in the combination arm than the single-agent arm. As a result, many of the women in the trial required dose reductions.
As a result of the capecitabine/docetaxel trial, this combination is now being evaluated as adjuvant therapy in a number of large international trials. The pharmacologic basis for this combination involves the fact that the enzyme responsible for activating capecitabine in tumor cells is up-regulated by the taxanes — specifically docetaxel.
lack of crossover in the xt trial
One concern about the capecitabine/docetaxel trial was whether or not it was a fair assessment of the efficacy of single-agent docetaxel. Would the same results have been observed if patients received sequential docetaxel followed by capecitabine? In fact, a significant fraction of patients in the docetaxel arm received capecitabine at the time of disease progression. If one could eliminate that subset of patients, I believe that single-agent docetaxel would probably have done even worse, in terms of survival, compared to the combination.
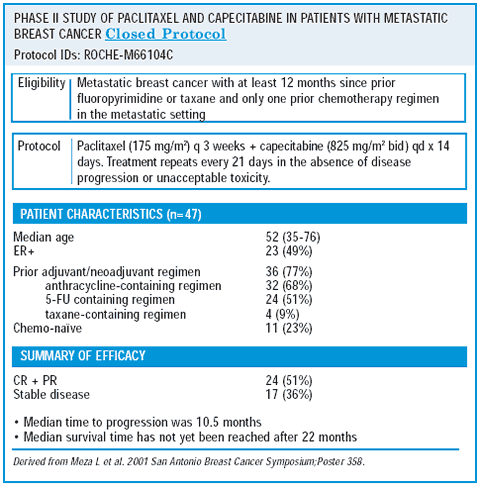
phase ii capecitabine/paclitaxel trial
The next natural question following the XT trial is, “Would similar results be achieved with a combination of capecitabine/paclitaxel?” We addressed this in a small phase II trial that demonstrated a 50% overall response rate and a 12% complete response rate for capecitabine/paclitaxel. Interestingly, the tolerability was somewhat better than that observed with capecitabine/docetaxel.
The regimen was well-tolerated — probably as a result of dose reductions. About 10-12% of the women experienced hand-foot syndrome. Although it is not fair to compare the results from a small phase II trial with those from a larger randomized trial, it might be worth further evaluating the capecitabine/paclitaxel combination in women with metastatic breast cancer.
combination versus sequential therapy in metastatic breast cancer
In women with metastatic breast cancer not enrolled on a protocol, we generally try to optimize single agents in a sequential manner. In those with rapidly progressing visceral disease — where a quick response is important — combination chemotherapy may be a reasonable alternative. Although response rates may increase with combination chemotherapy, almost inevitably there will also be more toxicity.
The capecitabine/docetaxel combination demonstrated a superior survival outcome, but few, if any, other combinations have ever shown a survival advantage. For the select patient with a good performance status, the combination of capecitabine/docetaxel or capecitabine/paclitaxel is perfectly reasonable. An alternative approach would be to use optimal single agents sequentially until disease progression.
There is a philosophical difference on how to approach women with metastatic disease. The real question is, “Are the side effects worth achieving a somewhat higher response rate?” Not to minimize the survival advantage — it is real — but there is a cost. However, if you know how to dose adjust, you can manage the patient’s side effects.

capecitabine as first-line therapy
Philosophically, sequential therapy involves the use of agents with the least toxicity and reasonable tumor control. An interesting question with sequential therapy would be, “Why not use capecitabine as first-line therapy followed by a taxane?” Even though capecitabine was approved as salvage therapy, this may be a reasonable approach. Since capecitabine demonstrated activity in women with refractory disease, there is no reason to believe it would not be effective as initial therapy. In select patients, those who will be compliant with an oral pill and do not want intravenous chemotherapy or those who progressed after adjuvant anthracycline-taxane therapy, capecitabine may be a reasonable choice.
Many patients will require dose adjustments when given the FDAapproved dose of capecitabine. At a reduced dose, patients may tolerate capecitabine, and the outcome may be similar to that with a taxane. The issue in first-line therapy is, “Can you make it equally effective with fewer side effects than the other alternatives available?” If capecitabine demonstrates efficacy, there would be a big advantage for many patients to an oral agent.
aromatase inhibitors: implications of the atac trial
Although the ATAC trial results are extremely interesting, it is still early in follow-up. The study evaluated only postmenopausal women, most of whom did not receive chemotherapy. As a result of the ATAC trial, women around the country are asking their doctors what they should be doing today. Numerous different scenarios can be envisioned. Women who have been on tamoxifen for six months, two years, three years, etc. are now asking their physicians if they should switch. The ATAC trial results do not address these situations.
The ATAC trial results are more relevant and germane to newly diagnosed postmenopausal women who are destined to receive adjuvant hormonal therapy. It would be fair and honest to discuss the ATAC results with caveats. It would be a consideration to put such a woman on an aromatase inhibitor. Using evidence-based medicine, I would likely prescribe the drug evaluated in the ATAC trial, anastrozole.
Even before the ATAC trial results were available, it was rational to use aromatase inhibitors as adjuvant therapy in women intolerant of tamoxifen. Based upon data from the metastatic disease trials, it was clear that aromatase inhibitors were very effective, as good if not superior to tamoxifen, as first-line therapy. Since we now have a 9,000-patient trial showing early on there is a clear advantage for anastrozole, there’s even more of a basis.
women about to start adjuvant hormonal therapy
In postmenopausal women with ER-positive breast cancer, anastrozole may be a consideration. The caveat being that we have thousands and thousands of patient years’ experience with decades of follow-up for tamoxifen, whereas the ATAC trial has only about two and one-half years of follow-up.
Another issue not yet answered by the ATAC trial is the long-term effect of anastrozole on the bone. If a young woman is rendered postmenopausal by chemotherapy, her long-term outcome may be 30-40 years in the future.
What would be the consequence of depleting calcium from her bones at a much earlier age compared to a woman who is 70? We need more data about bone fractures from the ATAC trial to understand exactly what we’re doing.
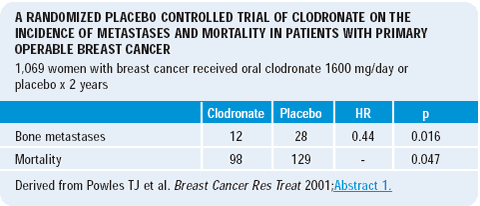
Trevor Powles’ clodronate data raise the possibility of the addition of a bisphosphonate to an aromatase inhibitor. Perhaps, an aromatase inhibitor plus a bisphosphonate will not only provide benefit in terms of cancer risk reduction but also in terms of maintaining overall quality of health. Bisphosphonates may potentially reduce disease progression or recurrence and also maintain bone density.
endocrine treatment strategies for premenopausal women
In premenopausal women still menstruating after chemotherapy, we discuss the option of ovarian ablation based upon the results from Intergroup trial 0101, but it is not the standard of care. However, in a young woman with a high risk of recurrence, it is worth discussing the potential of ovarian ablation. In women with low-risk, nodenegative disease, chemotherapy alone may be adequate to reduce their risk. The benefits of additional maneuvers beyond chemotherapy may be outweighed by the long-term side effects.
aromatase inhibitors plus ovarian ablation in premenopausal women
In a young woman with ER-positive, node-positive breast cancer, who does not become amenorrheic after chemotherapy, an LHRH agonist or ovarian ablation followed by tamoxifen may be considered. In that setting, theoretically it makes sense to consider an aromatase inhibitor. I have used an LHRH agonist plus an aromatase inhibitor in the metastatic setting, which is a very reasonable regimen in light of its tolerability.
differentiation between the aromatase inhibitors
I use all three aromatase inhibitors — anastrozole, letrozole and exemestane. As first-line therapy for metastatic breast cancer, the data are strongest for anastrozole and letrozole. Since the classes are not completely cross-resistant, a patient progressing after a non-steroidal agent — anastrozole or letrozole — could be considered for exemestane.
If you are driven by data, then anastrozole is clearly supported as the aromatase inhibitor of choice in the adjuvant setting. Letrozole may turn out to be similar to anastrozole, but the data are not yet available.
Select Publications
|
