| You are here: Home: BCU 3|2002: David W Miles, MD

Edited comments by Dr Miles
potential underestimation of capecitabine/docetaxel survival advantage
In a phase III trial by Nabholtz, docetaxel was compared to mitomycin-C plus vinblastine — our standard regimen for anthracycline failures at that time. Then we participated in the capecitabine/docetaxel trial, which clearly demonstrated a survival advantage. In advanced disease, we generally discuss the palliative benefits of chemotherapy, but over the last few years, the median survival for metastatic breast cancer has gone from 9 to 11.5 months up to 14.5 months with capecitabine/docetaxel. Since 27% of the women failing single-agent docetaxel received capecitabine, the survival benefit for the combination may have been underestimated.
xt versus t trial: treatment after progression on single-agent docetaxel
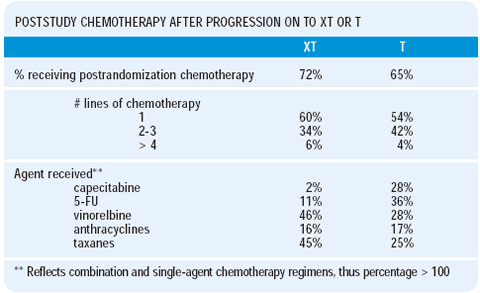
Increasingly, in clinical trials it is difficult to mandate therapy beyond crossover, which makes it difficult to interpret the attributable benefit of treatment up front. We looked at the poststudy chemotherapy administered to women progressing on single-agent docetaxel. About 28% of the women failing docetaxel received capecitabine. Interestingly, those women treated with capecitabine actually did better than those treated with other chemotherapeutic agents. The hazard ratio of dying was about half for those women receiving capecitabine compared to those receiving other chemotherapeutic agents. Also, 75% of the women had previously received 5-FU, yet there was still a great response rate for docetaxel/capecitabine compared to docetaxel alone.
Many would use vinorelbine after docetaxel failure, but the with that strategy is that both agents are spindle poisons. There are a few phase II studies of vinorelbine following taxane failure, with inconsistent results. When we compared vinorelbine to other chemotherapies following failure of docetaxel, vinorelbine was not associated with any difference in the hazard ratio of death, whereas there was a significant difference with capecitabine.
Derived from Miles D et al. 2001 San Antonio Breast Cancer Symposium;Poster 442.
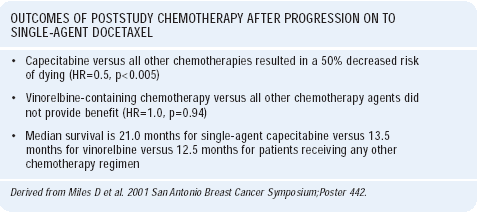
capecitabine/docetaxel: sequential versus combination therapy
The crucial question is whether combination therapy is better than sequential therapy. Since the progression-free and overall survival curves separated very early in the original phase III trial by Joyce O’Shaughnessy, many are hesitant not to use the combination of capecitabine and docetaxel in women with a good performance status. You potentially risk losing the advantage of the combination if these agents are not used together. In women who may not tolerate the combination because of toxicity, it may be reasonable to use sequential rather than combination therapy.
Until we have a trial comparing sequential to combination therapy, we will not know the degree of benefit derived from combination therapy. Without the studies, we also will not know which drug should come first. Capecitabine is a very active drug in terms of raw response rates, although it may not be quite as effective as docetaxel. However, there is a group of women in whom you may want to consider capecitabine as a single agent. Until we obtain data on sequential therapy, we have results demonstrating that the capecitabine/docetaxel combination is better than single-agent docetaxel.
capecitabine/docetaxel inmetastatic breast cancer
In young, fit women with visceral disease for whom survival is a serious issue, the capecitabine/docetaxel combination should be considered as a nonprotocol regimen. For women who are less healthy and perhaps older, some may not consider the combination as attractive. In the phase III trial, almost two-thirds of the women had dose reductions. The 75 mg/m2 of docetaxel and 1,250 mg/m2 of capecitabine (B.I.D. for two weeks, then one week off) may be a difficult regimen for some women, and it may need to be dosereduced or one might consider a single agent. It’s a choice that you and a woman must make together.
capecitabine/docetaxel toxicities
With capecitabine and docetaxel, there are nonoverlapping toxicities. Myelosuppression with capecitabine is negligible, but there is GI toxicity and the hand-foot syndrome. Capecitabine’s clearance is dependent on renal function. In women over 60 years of age, it is necessary to start at 1,000 mg/m2 b.i.d.— two weeks followed by one week off. This may also be the case for younger patients receiving concomitant docetaxel. The maximum tolerated dose for docetaxel is different around the world. As evidenced by the number of women in the phase III trial with dose reductions, docetaxel 75 mg/m2 in combination with capecitabine was difficult to administer, but the toxicity for this combination was manageable. Despite the dose reductions, there was still a survival benefit. People are anxious about the combination because of the toxicity element. But if you are interested in increasing survival in this group, I think capecitabine/docetaxel has to be considered the standard of care.
mechanism of action of capecitabine
Capecitabine’s mechanism of action probably goes beyond that of 5-FU. Thymidine phosphorylase (TP) concentrations are higher in tumor cells than normal tissue. Additionally, drugs such as docetaxel and paclitaxel probably induce TP. Recent studies looking at the scheduling of capecitabine/docetaxel in animal models have been reported. A Japanese group looked at different schedules of the two drugs. In fact, giving capecitabine on days 1-14 and docetaxel on day eight instead of day one seemed better. Since docetaxel was used later and theoretically TP was not upregulated until later, TP may not be the whole story — there may be something else going on. There is certainly some added value for capecitabine compared to 5-FU.

role of single-agent capecitabine
Single-agent capecitabine may be considered before a taxane — perhaps in older women, or those with a poor performance status or non-life-threatening disease. In a woman who is symptomatic or whose liver function is impaired, the goal is to maximize the response rate. So, combination therapy may be considered. An interesting trial would be the comparison of capecitabine/docetaxel to docetaxel alone or capecitabine alone. More toxic treatment is not necessarily better.
Select publications
|
