| You are here: Home: BCU 3|2002: Jack Cuzick, Phd

Edited comments by Dr Cuzick
unblinding the atac trial data
As the independent statistician, I was the only person able to link the data from the trial with the treatment code and to provide the coded results to the data monitoring committee. As a result, I was the only person who saw the unblinded data as it evolved, and it was very, very exciting. As the results began to appear six months ago, it has been very difficult to keep quiet. Tamoxifen has been the standard endocrine treatment for breast cancer for 30 or 40 years. Now a new treatment looks better, not only in terms of efficacy but also safety. Of course, this is still early data — with two and onehalf years of follow-up — but we are seeing a striking improvement in recurrence rates and a reduction in contralateral breast cancer with anastrozole.
atac results — efficacy
The most striking finding is that the combination of anastrozole and tamoxifen is no better than tamoxifen alone. Evidence in advanced disease indicates that aromatase inhibitors are more effective than tamoxifen, so although it is really gratifying to see the same results in the adjuvant setting, it is not unexpected. No one had a clear idea of what would happen in the combination arm. Apparently, once the estrogen receptors are saturated with tamoxifen — as occurs in postmenopausal women — reducing estrogen levels with an aromatase inhibitor has no effect on the disease.
In estrogen receptor-positive women, there was almost a 25% reduction in recurrence rates in the anastrozole arm compared to tamoxifen arm. Tamoxifen produces a 40% reduction in recurrence rates compared to controls, and to obtain an additional 25% reduction beyond tamoxifen with an agent that has a more favorable side-effect profile is an enormous step forward.
Anastrozole demonstrated almost a 60% reduction in contralateral breast cancer rates over tamoxifen. Tamoxifen itself provides a 50% reduction compared to no treatment. Therefore, we are talking about a potential 80% reduction in new breast cancers. If this were the case in the prevention setting, it would be fantastic.
atac results — risks
BONE MINERAL DENSITY
The fracture rate was increased from roughly five percent in the tamoxifen arm to seven percent in the anastrozole arm. Of course, tamoxifen has a beneficial effect on bones. Probably about half of the difference in fractures may be attributable to the reduction in fracture rates associated with tamoxifen. The other half of the difference in fractures may be related to anastrozole’s negative effect on bone. In a few months, there will be more data available from the bone subprotocol.
I think the effects on bone will be reversible, but if we will be giving aromatase inhibitors for a long time in the adjuvant setting, this will emerge as one of the key issues. The ATAC trial will go on for five years, but there is discussion about re-randomizing at five years to go on for ten years. The issue of how to manage bone loss is going to become paramount.
In the forthcoming IBIS II prevention trial — comparing anastrozole to tamoxifen and placebo — a subgroup of women will be randomized to receive vitamin D and calcium supplements or placebo. If vitamin D and calcium fail, then we will need to look at the bisphosphonates.
ARTHRALGIAS
There was a six percent increase in arthralgias associated with anastrozole. The arthralgias did not have a significant impact on the dropout rate from the trial. We need to look more carefully at the severity and the duration of the arthralgias.
WEIGHT GAIN
All of the evidence that is reliable indicates that there is no weight gain associated with tamoxifen, but in ATAC there was less weight gain with anastrozole. I think it is too early to look at that data — we have just touched the surface of really exploring a lot of these somewhat surprising new side effects.
GYNECOLOGIC
The endometrial cancer data are very striking — there were 11 cases in the tamoxifen arm compared to three in anastrozole arm. There was no evidence of endometrial cancer being a problem with anastrozole. There was a large difference in vaginal bleeding. Vaginal bleeding increased with tamoxifen, whereas anastrozole demonstrated an 80% reduction. Although not evident in the metastatic trials, most clinicians believe that anastrozole produces fewer vasomotor symptoms than tamoxifen. The ATAC trial demonstrated that anastrozole was associated with fewer hot flashes than tamoxifen.
STROKE/THROMBOEMBOLIC EVENTS
The stroke rate was reduced by more than 50% with anastrozole. This was highly significant and potentially very important. We saw an increase in stroke from tamoxifen in the P-1 trial, and people were still a little skeptical as to whether that was real or not. This is indirect confirmation that it is higher in the tamoxifen arm than it is in the anastrozole arm. This may be a side effect of tamoxifen.
continuation of the atac trial
Most of the women enrolled on the ATAC trial are in their third or fourth year of treatment. Clearly, the women in the study will be informed of these results, and they will be asked to consent to continue in the trial. Women who want to find out which drug they are receiving will be informed and dropped from the trial. It is believed that the combination arm will be discontinued. It is likely that the women on the combination arm will be able to choose between tamoxifen and anastrozole. It is also possible that they may be randomized between the two treatments.
I hope this trial will have a re-randomization to look at duration of therapy — five versus ten years of anastrozole. I think the duration of therapy will emerge as a key issue. Probably longer is going to be better than shorter. Five years may just be the beginning — ten years may be best. Key issues will be the bone problems and possibly cognition.
differentiation between the aromatase inhibitors
There will be much discussion as to whether these results are applicable to the other aromatase inhibitors — letrozole and exemestane, but there may be subtle differences in their side-effect and phamacokinetic profiles.
adjuvant trials on the horizon
There are a number of interesting options for new adjuvant trials. One that is particularly attractive is the use of the pure antiestrogens — drugs like fulvestrant. An interesting question is whether fulvestrant has something to offer in combination with an aromatase inhibitor.
Another area where there is a need for trials is in premenopausal women. The aromatase inhibitors are better than tamoxifen in postmenopausal women, but one-third of the breast cancer cases occur in premenopausal women. In premenopausal women, the LHRH agonists are an option in those with ER-positive disease — they are as effective as chemotherapy. LHRH agonists render a woman postmenopausal, and at that stage, the addition of an aromatase inhibitor could be considered. That is an interesting question. Should we be using LHRH agonists plus an aromatase inhibitor as a more complete method to deprive tumors of estrogen? Is an LHRH agonist plus an aromatase inhibitor more effective than chemotherapy?
prevention trials in premenopausal women
In order to understand how lifestyle modifications can impact on breast cancer risk, we are conducting small trials in premenopausal women. Increasing epidemiological evidence suggests that exercise has a beneficial effect on cancer. Possibly, the dietary intake of soy products may have an effect on breast cancer. The other lifestyle factor is alcohol intake. Studies demonstrate that women who consume two drinks per day increase their breast cancer risk by about 30%. It is difficult to know whether this is a causal effect.
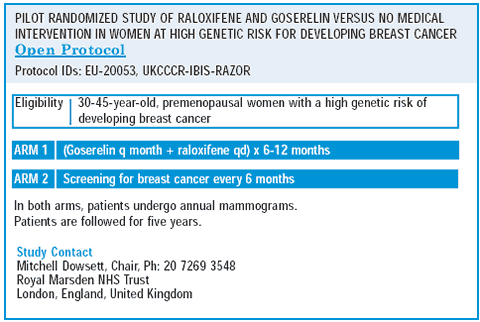
There is interest in looking at the LHRH agonists with some sort of add-back. We are conducting pilot trials evaluating an LHRH agonist plus raloxifene to protect the bone. We need to find an addback to control symptoms. There are lots of exciting possibilities — an LHRH agonist in combination with anastrozole plus some sort of bisphosphonate.
Select publications
|
