| You are here: Home: BCU 7|2003: Howard A Burris III, MD
Edited comments by Dr Burris
Phase II trial of weekly paclitaxel/carboplatin/trastuzumab
Trial design
Patients received double doses of induction trastuzumab for eight weeks and were then assessed by CAT scan. Those who progressed were taken off trastuzumab and started on paclitaxel/carboplatin. Those who responded to the induction trastuzumab continued on trastuzumab for an additional eight weeks before starting paclitaxel/carboplatin.
The idea was to give approximately six cycles of the three-drug combination. As the trial evolved, physicians began to continue maintenance trastuzumab at the completion of chemotherapy. There were patients who maintained responses on trastuzumab alone for more than a year.
Dr Nick Robert's trial of every-three-week administration of paclitaxel/ carboplatin with weekly trastuzumab, and Dr Edith Perez's trial of weekly paclitaxel/carboplatin/trastuzumab - three weeks on, one week off - both had schedules similar to our trial. While our trial was weekly like the Perez study, the schedule was six weeks on, two weeks off, and patients did not receive trastuzumab during the break.
While we were initially criticized for that omission of trastuzumab for two weeks, we now know that it is probably not significant because of the long half-life of trastuzumab. In addition, in our trial, patients going to maintenance trastuzumab were allowed to receive it once every three weeks as that data emerged. We have learned that three weeks on, one week off, is a more convenient break pattern than the schedule used in our study, and all of our Phase II trials now follow that schedule.
Efficacy
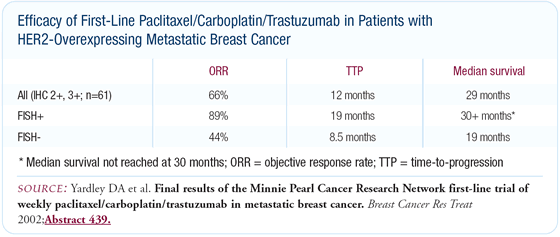
While we saw some responses with trastuzumab alone, when we put the three drugs together, the increase in response rate was impressive. Sixty-two patients enrolled in this trial, and 45 of the 62 received the three-drug combination. The response rate to induction trastuzumab was 15 percent, and it jumped to 70 percent when we added paclitaxel and carboplatin. The complete-response rate approached 20 percent, and a number of responding patients remained on therapy for quite some time.
The patients whose disease was resistant to trastuzumab and progressed did well with the paclitaxel/carboplatin combination. Because of the long half-life of trastuzumab, these patients probably still received some three-drug effect.
The response rate for this group was 60 percent - very similar to what has been reported. The progression-free survival was approximately 17 months, and the median overall survival was approximately 30 months. Patients with IHC 3+ and FISH-positive tumors received the most benefit from the combination, and their overall survival was approximately three years.
Ninety percent of patients on this trial benefited by attaining stable disease or better, and 70 percent experienced tumor shrinkage or better. With these high response rates, and as we've seen from Dr Robert's and Dr Perez's work, this three-drug regimen is probably as good as we first noted.
Toxicity
We wanted a nontoxic regimen and we certainly achieved that. There was no Grade 3/4 hemetologic toxicity, alopecia or mucositis.
Phase II data comparing weekly versus every-three-week administration of paclitaxel/carboplatin/trastuzumab suggests better tolerability with the weekly regimen. There isn't any response or survival data available yet, but I suspect that weekly administration will be at least as efficacious as every three weeks.
When discussing this with patients, I generally recommend the weekly regimen unless they have a long distance to travel or some other unique circumstance that makes that difficult. The weekly regimen has a very mild toxicity profile.
It surprises people that adding both paclitaxel and carboplatin to trastuzumab rather than adding one or the other doesn't seem to adversely affect the toxicity profile. People often say, "Surely there's more toxicity with a triplet than single-agent trastuzumab," but that doesn't seem to be the case with the weekly therapies. This is one reason we chose to err on the side of being a little more aggressive.
Future investigation of the taxane/carboplatin/trastuzumab combination
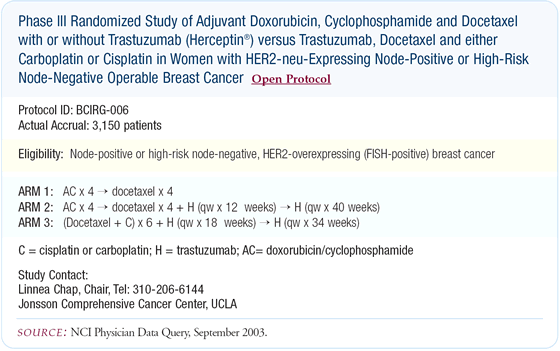
We've been surprised how quickly the taxane/platinum/trastuzumab combination has moved into the adjuvant setting. The adjuvant BCIRG trial utilizing this combination is accruing over 100 patients a month. Accrual will probably be completed by early next year, and we'll see how this triplet fares in the adjuvant setting.
In addition, everyone wants to know how the carboplatin/trastuzumab combination would have fared in metastatic disease, so we're going to do a pilot study in which patients receive carboplatin/trastuzumab for at least two cycles, and a taxane will be added in the nonresponders. Dr Mark Pegram published the first cisplatin/trastuzumab trial in 1998, which had a 24 percent response rate for second- and third-line therapy in metastatic breast cancer. I suspect our trial will show that the benefit of adding a taxane will offset any additional toxicity.
Page 1 of 2
Next
|
