| You are here: Home: BCU 7|2003: Richard
M Elledge, MD
Edited comments by Dr Elledge
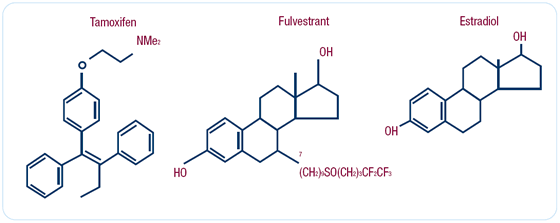
Effects of fulvestrant on estrogen receptor biology
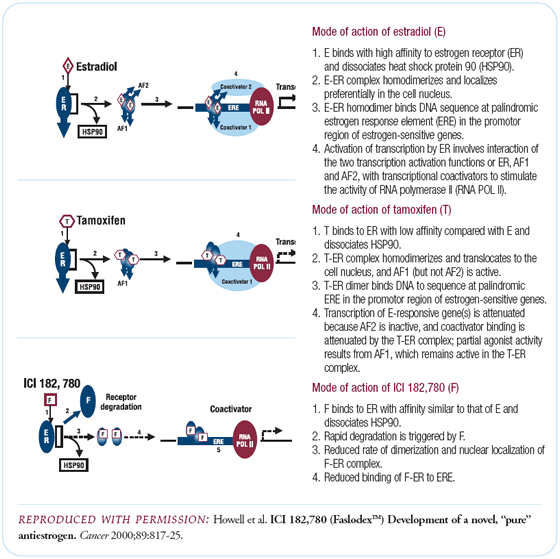
The estrogen receptor (ER) is a transcription factor that turns
on and off certain genes important for regulating tumor cell growth
and survival. Tamoxifen acts by binding this ER, resulting in partial
stimulation and partial blockade of the receptor, depending on
the context. This partial agonist/antagonist effect causes some
of the side effects of tamoxifen, such as endometrial growth, and
may also limit the full therapeutic application of interacting
with the receptor.
Fulvestrant is in a different class of molecules than tamoxifen.
While tamoxifen is a nonsteroidal molecule, fulvestrant is a steroidal
molecule and an analogue of estradiol. This agent does not appear
to have any stimulatory effect - it completely inhibits ER action.
In human breast cancer cells in the laboratory, fulvestrant decreases
the level of ER inside the tumor cell by 80 percent to 90 percent - and
many times below the level of detectability. Unlike tamoxifen,
in laboratory models, fulvestrant shows no uterine stimulatory
effect, which gives us promise that we won't have the endometrial
cancer problem. In addition, it inhibits the growth of human breast
cancer in animal models more completely than tamoxifen or estrogen
withdrawal, which is equivalent to ovarian ablation. Fulvestrant
maximally shuts down a known growth stimulatory pathway in human
breast cancer, compared to tamoxifen, which only shuts it down
partially.
Tolerability data on fulvestrant
In the Phase II trial of fulvestrant and in the trial of women
with uterine fibroids, we didn't see any stimulatory effects on
the uterus as we see with tamoxifen. In fact, when given simultaneously
with tamoxifen or estrogen, fulvestrant blocks the uterine stimulation
caused by these agents. Fulvestrant also doesn't appear to cross
the blood-brain barrier as tamoxifen does.
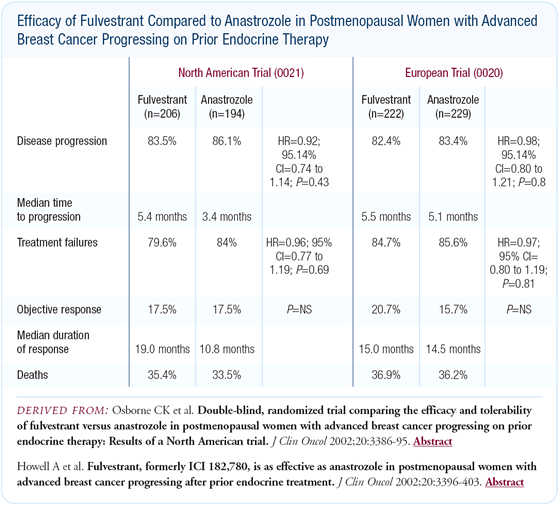
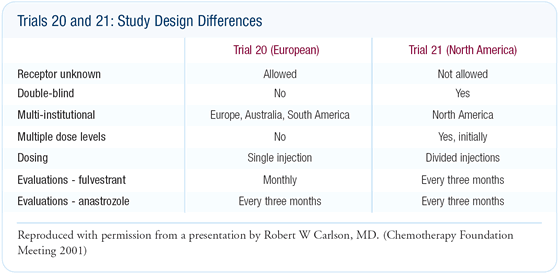
Randomized trials of fulvestrant versus anastrozole
The trials of fulvestrant versus anastrozole in patients progressing
on tamoxifen were large, well-executed studies - in contrast to
other hormone therapy trials done as recently as five years ago.
The fulvestrant versus anastrozole trials demonstrated that fulvestrant
is a very safe cancer therapeutic agent. There were virtually no
toxicities outside of background noise.
In terms of efficacy, these trials demonstrate that fulvestrant
is at least equivalent to a third-generation aromatase inhibitor,
currently our best endocrine agents for postmenopausal patients.
There were some hints that fulvestrant might be a little bit better
than anastrozole in terms of an increased duration of response,
but, overall, I believe they're equal.
In the European trial, the time to treatment failure in the two
arms was close to identical. In the American trial, the overall
objective response and clinical benefit rates were slightly higher
for fulvestrant, though not statistically significant.
The main difference between fulvestrant and anastrozole in the
American trial was the increased duration of response in the fulvestrant
arm. Not only was there a statistically significant improvement
from 10 months to 19 months, but this time difference is clinically
and humanly worthwhile in the metastatic setting. It tells us that
this agent might give us a bit of a boost in the adjuvant setting.
Page 1 of 2
Next
|
