| You are here: Home: BCU 7|2003: Rowan
T Chlebowski, MD, PhD
Edited comments by Dr Chlebowski
Terminology: Hormone replacement therapy versus
menopausal hormone therapy
The FDA and the NIH have removed hormone replacement therapy from
their lexicon. The preferred term is menopausal hormone therapy
or hormone therapy. Menopausal hormone therapy is probably more
descriptive, because hormone therapy could include oral contraceptives.
Women's Health Initiative (WHI) trial
The Women's Health Initiative trial randomized 16,608 healthy
postmenopausal women to conjugated equine estrogens plus medroxyprogesterone
acetate (PremproTM) or placebo.

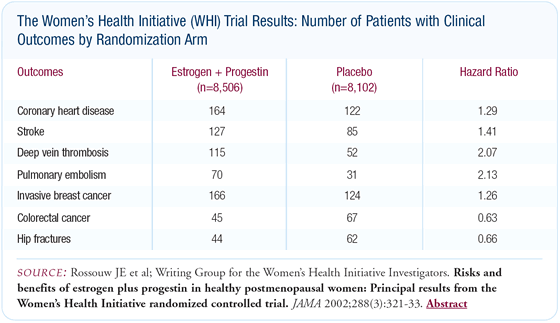
Overall results
The estrogen/progestin part of the WHI trial was prematurely stopped
after 5.2 years of follow-up because the global index, which involved
life-threatening conditions, suggested that there was risk associated
with estrogen plus progestin. The adverse effects included a 29
percent increase in coronary heart disease and a 26 percent increase
in breast cancer. The incidence of stroke and pulmonary embolus
was also substantially increased. On the favorable side, colorectal
cancer and hip fractures were significantly decreased by menopausal
hormone therapy. However overall, there were still 19 more adverse
events per 10,000 women per year of use of estrogen/progestin therapy.
Influence of menopausal hormone therapy on breast
cancer risk
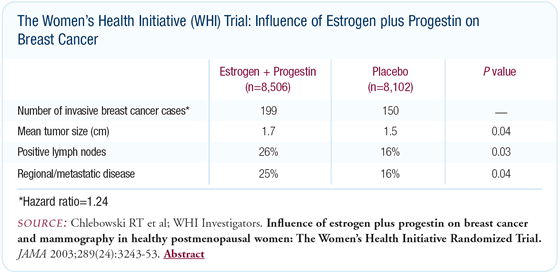
After a mean follow-up of 5.6 years, 349 cases of invasive breast
cancer were detected - 150 in the placebo group and 199 in the
estrogen plus progestin group.
In contrast to observational studies suggesting that the tumors
in the estrogen plus progestin group would be low grade and easy
to treat, we found these tumors to have identical histology and
grade, but a more advanced stage. The tumors in the estrogen plus
progestin group were larger (mean size of 1.7 versus 1.5 cm, P
= 0.04) and more likely to have positive nodes (26 percent versus
16 percent, P = 0.03), which were statistically significant findings.
The cancers that developed in the estrogen plus progestin group
included ER-positive and ER-negative cancers. The number of ER-positive
and ER-negative cancers increased by the same amount with hormone
therapy use. This indicates that estrogen plus progestin can stimulate
breast cancer growth.
Influence of menopausal hormone therapy on mammograms
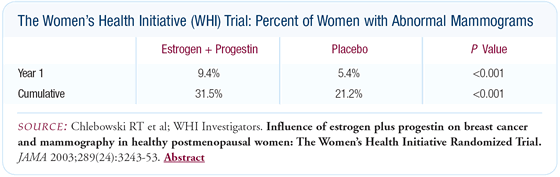
The mammogram results were probably the most surprising finding.
After one year of estrogen and progestin use, there was a four
percent absolute increase in the frequency of abnormal mammograms.
A woman would have a 1-in-25 chance of having an otherwise avoidable
abnormal mammogram by taking estrogen and progestin for just one
year.
The difference persisted throughout the study, and at the end,
the women in the estrogen plus progestin group had a 30 percent
chance of having an abnormal mammogram compared to about a 20 percent
chance for the women in the placebo group. Previously, it was believed
that there were no consequences from one or two years of estrogen
plus progestin use. Now, women must consider the 1-in-25 or 1-in-10
chance of having an abnormal mammogram.
The women in the estrogen plus progestin group had more advanced-stage
cancers diagnosed, even though they were the same grade, because
the abnormal mammograms hindered the diagnosis. For the first three
years there were fewer cancers diagnosed in the estrogen plus progestin
group than the placebo group, and it looked like the breast cancer
incidence decreased. In actuality, it was just harder to find the
cancers.
Page 1 of 2
Next
|
