| You are here: Home: BCU 7|2003: Rowan
T Chlebowski, MD, PhD
Applicability of results to younger women
Over 5,000 women in the WHI trial were between the ages of 50
and 59, and about 2,000 had moderate to severe vasomotor symptoms.
In the group of women 50 to 59 years of age, the increase in mammogram
abnormalities was the same as for the overall group. The WHI trial
raises questions about the short-term use of menopausal hormone
therapy, because it identifies an important side effect.
If a woman has moderate to mild estrogen-deficiency symptoms,
she must now decide whether 80-90 percent suppression of those
symptoms for a year or two is worth a 1-in-25 or 1-in-10 chance
of having an abnormal mammogram. This is a new thought process
for women considering menopausal hormone therapy.
For the woman with severe disabling symptoms, the chance of having
an abnormal mammogram, which doesn't necessarily mean she's going
to have breast cancer, is probably going to be a small consideration.
The actual chronic-disease risk associated with one, two or three
years of therapy for a woman 49 or 50 years of age is going to
be a very small number. The breast cancer risk for the overall
population involved eight additional breast cancers per year for
every 10,000 women receiving menopausal hormone therapy.
There were 19 avoidable life-threatening conditions (including
coronary heart disease and stroke) per 10,000 women per year of
estrogen plus progestin use. For women meeting the study criteria,
one in a hundred would have an otherwise avoidable life-threatening
event after five years of estrogen plus progestin use. The absolute
risk would be lower for 50-year-old women, but that is a statement
about the population, not the individual patient.
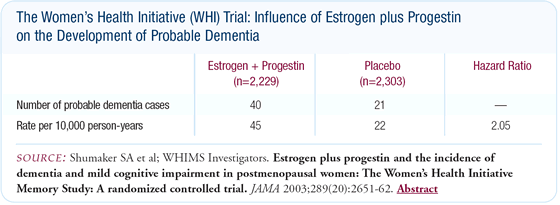
Influence of menopausal hormone therapy on dementia
There was a 180-degree turnaround associated with the ancillary
study results on dementia (see below). The prestudy assumption
was that we would see a substantial reduction in dementia. In actuality,
the subset of women 65 years of age and older had over a doubling
of dementia cases - from 21 to 40 cases -after five years of therapy.
We speculate that the arteriovascular effects, such as subclinical
stroke, may have raised the threshold so that the natural course
of dementia was evident sooner. The implication for women with
breast cancer is that other agents that cause arteriovascular events,
like chemotherapy or tamoxifen, may be associated with the same
increase in dementia. This phenomenon has not been carefully studied
with these other agents in the kind of detailed analysis that was
done in the WHI trial.
Selection of the estrogen and progestin
The WHI trial evaluated the estrogen and progestin type, dose
and schedule used by 85 percent of postmenopausal women with a
uterus until two years ago. They weren't natural estrogens and
progestins, even though there are some natural estrogens in conjugated
equine estrogens.
The Europeans use more estradiol and micronized progestin or natural
progestin. Whether those are going to be safer, we don't know.
The FDA made all combined estrogen and progestin products include
the same black-box warning that it required for conjugated equine
estrogens and medroxyprogesterone acetate.
Estrogen-alone arm in women with a prior hysterectomy
A separate WHI trial in just over 10,000 women with a prior hysterectomy
is evaluating an estrogen-alone arm. The data safety monitoring
board looks at that data twice a year. There is a little over six
years of follow-up, and by design, the trial results will be reported
at 8.5 years or in about two years. As of May 30, 2002, there was
no excess in breast cancer risk.
For several reasons, we can't be sure that it's just the progestin
causing the difference. First, women who have had a hysterectomy
are substantially different from women who have a uterus, in terms
of their medical history characteristics. Second, this trial is
smaller, and it may take longer to generate an equivalent number
of events. There are also biologic reasons to believe that we might
see something different in terms of breast cancer.
Dr Norman Boyd in Toronto has shown that breast density - especially
inherited breast density - is associated with an increased risk
of breast cancer. However, we don't know whether a short-term change
in breast density is associated with breast cancer risk.
It's intriguing that estrogen plus progestin substantially increases
breast density, whereas estrogen alone increases it much less.
That suggests there may be a difference. Based on the more recent
epidemiological data, one would think there would be less breast
cancer risk associated with estrogen alone, but we don't know.
Indications for menopausal hormone therapy
Menopausal hormone therapy is almost exclusively indicated for
the amelioration of hot flashes and vaginal symptoms. The FDA recommends
the use of the lowest possible dose and duration, although we don't
have information about the safety of those lower-dose schedules.
The FDA will hold hearings on the osteoporosis indication for
estrogen plus progestin combinations, which certainly are effective
in reducing the risk of hip fractures. However, since there are
alternatives available to reduce the risk of hip fractures, I'm
not sure an estrogen plus progestin combination is safe enough
for this indication.
Trends in menopausal hormone therapy use
The use of menopausal hormone therapy was increasing and then
leveled off after the Heart and Estrogen/Progestin Replacement
Study (HERS) reported no coronary heart disease benefits in women
with existing heart disease. At that time, there were about six
million women in the United States on an estrogen plus progestin
combination.
After the WHI report, the use of menopausal hormone therapy went
down significantly. Less than three million women are currently
using some kind of combined estrogen plus progestin therapy. Most
of the three million women who stopped taking an estrogen plus
progestin combination did so because they decided to stop, rather
than because their gynecologist told them to stop.
Over 80 percent of estrogen plus progestin use is short term in
perimenopausal women. A number of women, maybe one-fifth, are still
taking it for long-term chronic use. We'll see how those numbers
change after this most recent WHI trial report.
A decision-making approach to menopausal hormone
therapy
This situation appears analogous to the situation we commonly
face in a woman with an ER-negative tumor measuring less than one
centimeter, who is deciding whether to take chemotherapy for a
very small absolute benefit. I routinely try to project five years
into the future.
Will it be intolerable if she doesn't take the chemotherapy and
the tumor recurs? If she couldn't tolerate that and would say, "I
missed my chance," then she should take the chemotherapy. Alternatively,
if she projects herself five years from now and says, "I took the
chemotherapy and the tumor didn't recur - that was a bad decision," then
maybe she shouldn't take the chemotherapy. So, I give patients
those two future scenarios.
In a woman with mild or moderate menopausal symptoms, I would
say, "You may reduce the symptoms by 80 percent to 90 percent with
hormones, but you will have to deal with a 1-in-25 or 1-in-10 chance
of an abnormal mammogram. If you don't want to deal with an abnormal
mammogram, then don't start menopausal hormone therapy and see
what happens after a few months of just watching." If the patient
says, "I can deal with an abnormal mammogram," which almost certainly
is not going to be related to breast cancer, she should consider
taking the menopausal hormone therapy for a year or two.
Managing menopausal symptoms in patients with
breast cancer
I question some of the treatments we are using in breast cancer
patients with menopausal symptoms. For example, low-dose progestins
are effective in reducing symptoms. Medroxyprogesterone acetate,
interestingly, was found to be effective in women with resected
breast cancer; however, this is the same agent used in the WHI
trial. I would be very concerned about using progestins, especially
since many are pointing a finger at the progestin in this estrogen
and progestin mix.
The estradiol vaginal ring (Estring®) is a locally released
product that treats vaginal symptoms and atrophy. A study in the
Journal of Clinical Endocrinology and Metabolism demonstrated that
the estradiol vaginal ring use for one year increased HDL cholesterol
and decreased LDL cholesterol, the same as full-dose estrogen and
progestins.
If there is adequate absorption to change the lipid profile, it's
difficult to be completely sanguine about breast safety. In terms
of how to treat the patient with breast cancer and with menopausal
symptoms, we've identified a problem that will require more attention.
Page 2 of 2
Previous | Select publications
|
