| You are here: Home: BCU 7|2003: Vicente
Valero, MD
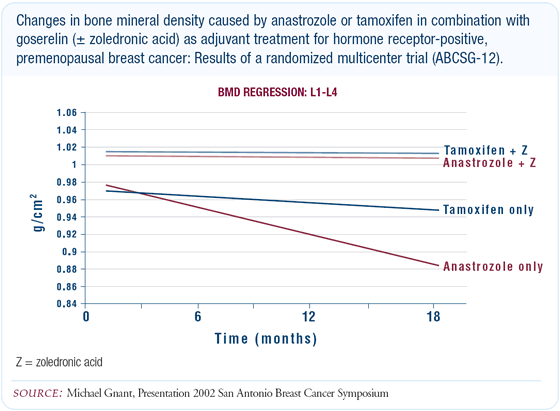
Adjuvant study of LHRH agonist plus tamoxifen
or anastrozole plus or minus zoledronic acid
At San Antonio, Dr Gnant's presentation of the Austrian study
data -comparing an LHRH agonist with either tamoxifen or anastrozole
with or without zoledronic acid - was very important in demonstrating
that a bisphosphonate could ameliorate the decrease in bone density
associated with hormonal therapy. Parenthetically, chemotherapy
results in a sharp decline in the production of estrogen, also
resulting in bone loss.
Clearly, we have to support our patients during treatment, just
as we do with growth factors for neutropenic-related events or
antiemetics for nausea. This is a very important issue for women
with breast cancer. The issue is: How do we incorporate the data
into clinical practice?
I assess bone density in postmenopausal patients receiving adjuvant
anastrozole. These women are already in menopause, so we know the
extent of their bone loss. The reduction in bone density from a
chronic therapy, such as adjuvant anastrozole, is different than
the acute bone loss from chemotherapy.
In a 60-year-old patient who has been in menopause for 10 years
without significant bone loss, I'm not convinced anastrozole will
produce a substantial decrease in bone density. On the other hand,
in a patient who already has osteopenia or osteoporosis, anastrozole
will likely exacerbate that.
You have to look at these patient populations differently. I assess
bone density and, if it is normal, I repeat it one year later to
determine whether or not to introduce a bisphosphonate; prophylactic
zoledronic acid may not be necessary.
Positive findings have been published in the New England Journal
of Medicine using intermittent zoledronic acid in patients with
osteoporosis. The next step is to compare this approach to the
conventional use of oral bisphosphonates in that setting. There
are studies done in conjunction with the ATAC trial that will give
us information about bone loss in patients on anastrozole versus
tamoxifen.
Bisphosphonates for the prevention of metastatic
disease
The limited data from trials of oral bisphosphonates are controversial.
Unfortunately, we don't have information using the more potent,
intravenous bisphosphonates. Randomized studies with zoledronic
acid are being initiated, but it will be a substantial period of
time before we have data.
Zoledronic acid infusions given at intervals of up to
one year produce effects on bone turnover and bone density
as great as those achieved with daily oral dosing with bisphosphonates
with proven efficacy against fractures, suggesting that an
annual infusion of zoledronic acid might be an effective
treatment for postmenopausal osteoporosis. 
EXCERPT FROM: Reid
IR et al. Intravenous zoledronic acid
in postmenopausal women with low bone mineral density. N
Engl J Med 2002;346:653-61. Abstract |
Preclinical data support the use of bisphosphonates for the prevention
of bone loss and bone metastases. Additionally, the landmark German
study by Diel and colleagues suggests that bisphosphonates may
also improve survival.
Increasingly, bisphosphonates are being utilized in women with
early breast cancer because we're using aromatase inhibitors in
that setting. How much secondary benefit these agents will provide
for prevention of bone metastases will be of significant interest
to physicians, and the best way to answer the question is with
a large, prospective, randomized study.
The primary obstacle is that we aren't able to select which patients
will have bone-only relapse. If we administer bisphosphonates to
everybody without targeting the patients likely to benefit, it
will be difficult to demonstrate a benefit. The absolute benefits
we have seen through the years are small.
Similarly, the benefit seen with taxanes ranges from an absolute
difference of one percent to eight percent. This means that the
minority of our patients are benefiting from the therapy. Ideally,
you'd like to look for this small percent of patients who benefit.
We are trying to study this at MD Anderson, using gene profiling
to try to prospectively confirm a specific gene profile to help
decide which patients should and should not receive a specific
therapy. This would allow us to spare toxicity and make therapies
more effective by improving the tools to select patients for treatment.
This is the direction the field is going, and it's very exciting.
Clinical advances with adjuvant taxanes
Several clinical trials have addressed the benefit of taxanes
in the adjuvant setting. The results from CALGB trial 9344 have
recently been published and demonstrate an improvement in disease-free
and overall survival with the addition of paclitaxel to AC chemotherapy.
BCIRG-001 - comparing TAC to FAC - also resulted in an improvement
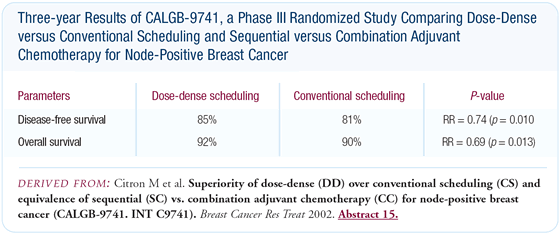
in disease-free survival. Most recently, CALGB-9741 documented
an improvement in disease-free and overall survival with dose-dense
AC and paclitaxel every two weeks with growth factor support.
Some physicians dismiss the findings from CALGB-9741, believing
that there is minimal clinical application of the results. I disagree.
Increasing the frequency of administration of AC and paclitaxel
from every three weeks to every two weeks, with filgrastim support,
clearly resulted in a substantial improvement in disease-free survival.
Interpretation of the results are controversial because CALGB-9741
was designed before the administration of weekly taxanes. Today
studies such as the Intergroup study ECOG-N9831 - for patients
with node-positive, HER2-positive disease - use weekly paclitaxel.
So, there has been a shift in the administration schedule of paclitaxel,
and some physicians question whether the improvement in disease-free
survival was due to increasing the density of paclitaxel, of AC
or of both. That issue remains unresolved, but a dose-dense approach
is an acceptable option for women with node-positive early breast
cancer.
Our results indicate interesting directions for further
research. For example, sequential dose-dense, single-agent
therapy could permit the rapid integration of new drugs into
therapeutic regimens, including biologic agents. Shorter
intertreatment intervals (i.e., beginning retreatment as
soon as the granulocyte count reaches 1,000/mL, rather than
at a fixed time interval) might be investigated. Quality
of life for patients receiving such treatments might also
be beneficially explored. Furthermore, research into the
biologic etiology of gompertzian growth and the molecular
mechanisms of its perturbation could be used to hypothesize
new, empirically verifiable dose-schedule manipulations.  EXCERPT
FROM: Citron ML et al. Randomized
trial of dose-dense versus conventionally scheduled and sequential
versus concurrent combination chemotherapy as postoperative
adjuvant treatment of node-positive primary breast cancer:
First report of Intergroup trial C9741/Cancer and Leukemia
Group B trial 9741. J Clin Oncol 2003;21(8):1431-9.
Abstract EXCERPT
FROM: Citron ML et al. Randomized
trial of dose-dense versus conventionally scheduled and sequential
versus concurrent combination chemotherapy as postoperative
adjuvant treatment of node-positive primary breast cancer:
First report of Intergroup trial C9741/Cancer and Leukemia
Group B trial 9741. J Clin Oncol 2003;21(8):1431-9.
Abstract |
Capecitabine/docetaxel (XT) in the treatment
of breast cancer
Dr O'Shaughnessy's study of women with metastatic breast cancer
demonstrated that the combination of capecitabine/docetaxel - compared
to docetaxel alone - resulted in improved response rate, time to
progression and survival.
The dosing and scheduling of the combination are controversial
and remain to be defined. In the XT trial, the drugs were given
simultaneously on day one. It's possible that upregulating TP with
a taxane should be done before introducing capecitabine, and perhaps
lower doses will result in the same benefit. If you want to utilize
aggressive therapy, the combination in the XT trial was superior,
and the quality of life wasn't impaired compared to the sequential
approach.
We're evaluating capecitabine/docetaxel as neoadjuvant and adjuvant
therapy. We're conducting a randomized study of weekly paclitaxel
for 12 cycles followed by FAC for four versus docetaxel/capecitabine
for four followed by FAC for four. If a patient with Stage II breast
cancer (or greater) has an intact tumor, she will receive primary
chemotherapy. If she has undergone locoregional therapy, she'll
be randomized for adjuvant therapy.
The addition of capecitabine to docetaxel resulted in
a 23 percent reduction in risk of death compared with docetaxel
alone, with an increase in median survival of three months.
The survival benefit with capecitabine/docetaxel combination
therapy was seen early in the course of treatment and persisted
throughout the study. The survival difference can clearly
be attributed to the addition of capecitabine, because patients
in the combination arm received a lower dose of docetaxel,
and there was no excess death rate due to administration
of full-dose docetaxel. A high proportion of patients in
both treatment groups received poststudy chemotherapy, and
the incidence of poststudy chemotherapy administration was
balanced between the two treatment groups (70 percent versus
63 percent with combination therapy and single-agent docetaxel,
respectively).
 EXCERPTED
FROM: O'Shaugnessy J et al. Superior
survival with capecitabine plus docetaxel combination therapy
in anthracycline-pretreated patients with advanced breast
cancer: Phase III trial results. J Clin Oncol 2002;20:2812-23.
Abstract EXCERPTED
FROM: O'Shaugnessy J et al. Superior
survival with capecitabine plus docetaxel combination therapy
in anthracycline-pretreated patients with advanced breast
cancer: Phase III trial results. J Clin Oncol 2002;20:2812-23.
Abstract |
Page 2 of 2
Previous | Select publications
|
