 |
Editor’s Note |
|
Images from the retrospectiscope
and prospectiscope |
A recent tumor panel discussion I moderated at the 2005 Miami Breast Cancer Conference included a fascinating case that foreshadows a major paradigm shift in this disease. The 43-year-old premenopausal woman whose case was presented by her South Florida-based medical oncologist was diagnosed several years ago with a small lesion on mammography. This proved to be a 0.7-cm, ER/PR-positive, HER2-negative, infiltrating ductal carcinoma. The axillary node dissection was negative.
After much discussion about whether any adjuvant therapy was indicated in what was considered a low-risk situation based on tumor size and histology, this woman elected to receive tamoxifen alone. Two years later, bone metastases were detected and a biopsy confirmed recurrence. The patient is now receiving palliative systemic management.
What is particularly interesting about this case is that the patient’s medical oncologist retrieved the original tumor block and was able to obtain an Oncotype DX™ assay, which revealed a high recurrence score. (This was done for academic purposes without charge to the patient.)
In retrospect, had this assay and the results from the latest relevant research data set been available at the time of this patient’s initial diagnosis, and had the patient received relatively nontoxic adjuvant chemotherapy (ie, CMF or M 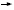 F), the likelihood of her relapsing would have decreased by about 75 percent. Put in more direct terms, three out of every four women and their families in this situation might have been spared the profound sadness and infirmity associated with metastatic breast cancer. F), the likelihood of her relapsing would have decreased by about 75 percent. Put in more direct terms, three out of every four women and their families in this situation might have been spared the profound sadness and infirmity associated with metastatic breast cancer.
In this issue of Breast Cancer Update for Surgeons, we again visit Soon Paik, one of the brilliant architects — along with Genomic Health medical oncologist Steven Shak — who developed perhaps the most important breast cancer clinical research database in the last decade. I interviewed Soon at the 2003 San Antonio Breast Cancer Symposium where he initially presented the results from the NSABP initiative with Genomic Health, and I again chatted with him at this past December’s meeting. Both visits were quite memorable, and the most recent interview is included in this program.
Every physician treating breast cancer patients must fully understand this research because it has direct and important implications for daily patient care. Put very simply, women with estrogen or progesterone receptor-positive, node-negative tumors must be informed of the option of having an Oncotype DX assay performed on their tumor tissue. Until recently, many insurers and governmental reimbursement agencies balked at paying the $3,000 plus tab for this test, but with the emergence of Soon’s most recent findings, these bureaucrats will now be standing in line to pay.
Taken at a pure monetary level, the assay has now become the best investment in town because fewer patients will receive costly adjuvant chemotherapy up front, and less resources will be invested in palliative regimens later on. The human savings are even more astonishing and impossible to quantify.
Every day I hear about more personal situations in which this assay has been utilized effectively, not only to identify patients who will benefit from receiving chemotherapy but also patients who will not benefit. A case in point is the woman who transcribes the research leader interviews for this audio series. This petite 60-year-old very talented ball of fire has a completely untouched head of dark, wavy hair, mainly because of the Oncotype DX assay.
In December, while recuperating from breast cancer surgery, this woman — who is a true master of her trade — decided to return to work, and one of her first assignments was to transcribe Dr Paik’s interview. Having recently learned of her pathology results (ER/PR-positive, node-negative), she instantly recognized the personal relevance of Soon’s findings and spoke with her surgeon, who called me. After hearing of these latest data, he ordered the assay.
When the results suggested a low chance of recurrence, the patient and her surgeon decided to forego adjuvant chemotherapy. Now, before beginning her day deciphering rapid-fire talkers and investigators with accents from all over the globe, she munches a daily anastrozole pill that will further reduce her already low risk of recurrence.
The landmark research collaboration that spared this woman the rigors of adjuvant chemotherapy is just the first of what is likely to be a long series of similar studies with many other new and perhaps less costly predictive assays. The NSABP initiative utilized a new technology developed by Genomic Health to evaluate a 21-gene panel in paraffin-embedded tumor tissue. Essentially, the Oncotype DX assay was able to determine that about half of the patients were in a favorable risk stratum with less than a seven percent chance of recurrence on tamoxifen. These numbers are likely to be even lower with aromatase inhibitors (AIs), as discussed in this program by Tony Howell, principal investigator of the ATAC trial.
The most recent NSABP data set presented by Soon documented a profound effect of adjuvant chemotherapy on the risk of relapse in the higher-risk subset. One might imagine that today, if the 43-year-old patient presented at the 2005 Miami Breast Cancer Conference tumor panel discussion had been identified as being in the high-risk category, she might not only have received adjuvant chemotherapy, but perhaps more aggressive adjuvant endocrine therapy than just tamoxifen. Specifically, ovarian ablation or suppression plus tamoxifen might have been considered for this patient, or even the option of an aromatase inhibitor added to ovarian suppression, although minimal clinical trial data currently support this strategy.
In general, a variety of clinical trials continue to demonstrate an advantage for AIs over tamoxifen in postmenopausal women, either in the up-front setting — as with anastrozole in ATAC — or after two or five years of adjuvant tamoxifen, as documented in a number of trials. The next generation of studies is addressing the role of AIs with ovarian suppression in premenopausal patients and the optimal duration of adjuvant AI therapy.
During my oncology fellowship in the late 1970s, tamoxifen became the beacon of targeted therapy, first in metastatic disease, then as adjuvant therapy for invasive disease and then DCIS, and finally as chemoprevention in women at high risk.
Now, after decades in the unique role as the optimal available endocrine therapy, this amazing SERM has finally been displaced by aromatase inhibitors for postmenopausal women. Tony Howell also reviews evolving research demonstrating that while the three available aromatase inhibitors have similar antitumor effects, interesting differences are beginning to emerge related to the risk of side effects and long-term sequelae, including cardiovascular disease.
The other two speakers interviewed in this program, surgeons Mel Silverstein and Chip Cody, discuss recent developments in local therapy for breast cancer, particularly related to decreasing morbidity. Partial breast irradiation and sentinel node biopsy are particularly noteworthy. While these strategies may not affect tumor control, the morbidity and inconvenience to the patient are considerably decreased compared to conventional breast irradiation and axillary node dissection.
It is always distressing to consider patients diagnosed just prior to research advances because these people have narrowly missed the opportunity to benefit. However, when one considers such cases retrospectively, we are better able to understand the human value of clinical and translational research, and thinking prospectively, we have hope that cancer therapy will become increasingly effective with fewer side effects and long-term complications for our patients.
— Neil Love, MD
NLove@ResearchToPractice.net
Select publications
Coombes RC et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004;350(11):1081-92. Abstract
Goss PE et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349(19):1793-802. Abstract
Howell A et al; ATAC Trialists’ Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365(9453):60-2. Abstract
Paik S et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817-26. Abstract
|

