Soonmyung Paik, MD |
EDITED COMMENTS |
 Oncotype DX™ multigene assay as a prognostic factor in patients treated with tamoxifen Oncotype DX™ multigene assay as a prognostic factor in patients treated with tamoxifen
At the 2003 San Antonio meeting, when I presented the initial data on this assay, Dr Kent Osborne raised a question about whether the recurrence score is a prognostic or predictive factor.
Frankly, we didn’t really care, as long as it’s a prognostic factor in that specific setting of tamoxifen-treated patients, so that we can identify a cohort of patients who don’t need chemotherapy.
Using the NCCN or St Galen criteria, in the tamoxifen-treated cohort in NSABPB-14 we would identify about eight percent of patients who don’t need chemotherapy. If we use the Genomic Health assay, we identify 50 percent — a huge increase in the number of patients categorized as low risk and not requiring chemotherapy.
The median 10-year distant failure rate was about 6.8 percent in patients who received tamoxifen with a low recurrence score, but the individual risk ranged from three percent to 12 percent, which is another strength of this test.
Although the NSABP usually refrains from subset analyses, supplementary information accompanying the New England Journal of Medicine article (Paik 2004a) details several subset analyses. Questions arose about whether the Oncotype DX assay would work in patients with tumors smaller than one centimeter, patients older than 60 years and other subsets in which the statistical power is much less; however, the overall trends seem to show that the assay works in every subset we evaluated. It always seemed to divide patients into low- or high-risk categories, regardless of histology grade or tumor size.
Prediction of response to chemotherapy with Oncotype DX assay
NSABP-B-20 included women with node-negative, ER-positive disease. It was a three-arm design, and patients were randomly assigned to tamoxifen alone or tamoxifen concurrent with either CMF or methotrexate followed by 5-FU. Our study was a retrospective analysis of that completed trial.
We repeated the Oncotype DX assay on the tamoxifen arm to ensure the assay was reproducible, and we demonstrated that it is reproducible, which is encouraging for a clinical assay. We also evaluated the NSABP-B-20 chemotherapy arms to address whether the assay predicted chemotherapy responsiveness. We went into that study with an a priori hypothesis based on data presented at the 2004 ASCO meeting by Dr Luca Gianni’s group in Milan, evaluating samples from a neoadjuvant trial of paclitaxel and doxorubicin.
They demonstrated a correlation between the Genomic Health recurrence score and pCR rate (Gianni 2004). The higher recurrence rate correlated strongly with the higher pCR rate. The overall pCR rate was approximately 25 percent in the patients with high-risk disease, and no pCR occurred in patients with low-risk disease.
We hypothesized that the benefit from chemotherapy in NSABP-B-20 would be almost negligible in patients with low-risk disease and high in patients with high-risk disease. The results of this study are actually quite striking and unlike anything I’ve ever seen (Paik 2004b).
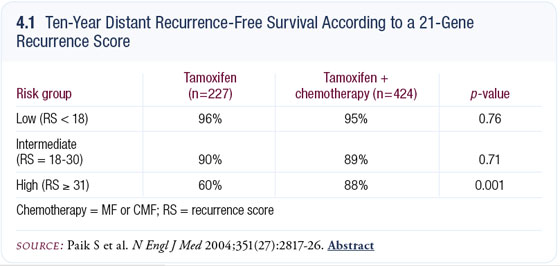
The absolute benefit from chemotherapy is actually negative in the low-risk group and zero in the intermediate-risk group. In the high-risk group, the absolute improvement in distant recurrence at 10 years is 28 percent, or a relative risk reduction of 75 percent (4.1).
The data in the low-risk group are, in a sense, not relevant, because the baseline risk after tamoxifen is so low — 6.8 percent — so it’s a moot point of whether they need chemotherapy or not. In the intermediate-risk group, the confidence interval overlaps with one, so whether patients with intermediate-risk disease gain any benefit or not remains a question.
Implications of the Oncotype DX assay study results
These data provide an important paradigm shift in the way we think about clinical trial design and patient management. So far, in most clinical trial designs, we presume that the proportional benefit or incremental gain would be the same degree in patients with low-risk and high-risk disease. All statistical sample size calculations are based on that assumption, but now we have to change that.
This data set also forces us to think about new clinical trial designs in which we preselect patients who are at high risk, because those are the patients who will benefit from chemotherapy. We already knew from other studies that ER-positive patients do not benefit much from chemotherapy. In the neoadjuvant trials, the pCR rate is much lower in ER-positive tumors. This study definitely shows that based on genes related to proliferation or estrogen receptor, we can actually select patients who are the best candidates for chemotherapy trials.
Potential impact of Oncotype DX on Ravdin’s Adjuvant! model
Peter Ravdin notes that in the Adjuvant! program, the relative benefit of chemotherapy is presumed to be equal for patients at higher and lower risk, but it’s likely that the estimation of chemotherapy benefit in the group with low-risk disease is an overestimation. Conversely, the benefit in the group with higher risk disease may be underestimated. I believe our studies with Oncotype DX demonstrate this, and Ravdin’s model may need to be slightly modified.
My prediction is that when people see these data, they will want the assay performed because nobody wants to receive chemotherapy when it will not work. I’m sure a lot of competing assays are being developed that will claim to do the same thing. As a clinical trial group, we are interested in supporting all of those studies. In my lab, we are trying to develop competing assays that will be less expensive and based on factors such as histology and estrogen receptors. We must demonstrate in a clinical study in a stepwise fashion as we did with Genomic Health that a marker is reliable and reproducible clinically so that patients will have confidence in the results.
Select publications
 |
Dr Paik is the Director of the Division of Pathology at the National Surgical Adjuvant Breast and Bowel Project in Pittsburgh, Pennsylvania. |
|

