|

| Tracks 1-15 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
ECOG-E2100: Paclitaxel with
or without bevacizumab as firstline
therapy for metastatic
breast cancer |
| Track 3 |
Clinical use of bevacizumab |
| Track 4 |
Nanoparticle albumin-bound
(nab) paclitaxel as single-agent
and combination therapy |
| Track 5 |
Continuation of bevacizumab
after disease progression |
| Track 6 |
Clinical benefits and efficacy
of nab paclitaxel |
| Track 7 |
Side-effect profile of
nab paclitaxel |
| Track 8 |
Estimated efficacy of nab
paclitaxel compared to
standard paclitaxel |
| Track 9 |
Strategies to prevent taxaneassociated
neuropathy |
|
| Track 10 |
Advantage of avoiding
premedication with
nab paclitaxel |
| Track 11 |
Role of nab paclitaxel in the
metastatic and adjuvant settings |
| Track 12 |
Management of ER/PR-negative,
HER2-negative metastatic
disease |
| Track 13 |
Selection of first-line
chemotherapy for the
treatment of metastatic
disease |
| Track 14 |
Combination versus single-agent
therapy in the treatment
of metastatic breast cancer |
| Track 15 |
Efficacy of capecitabine
versus taxane therapy in
the metastatic setting |
|
|
Select Excerpts from the Interview*
 Track 2 Track 2
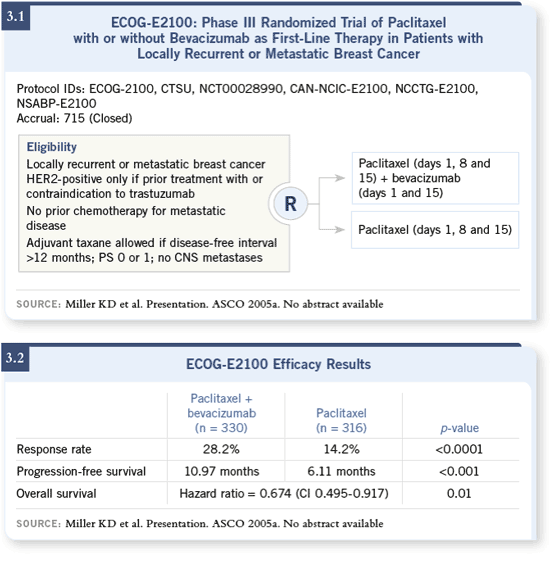
 DR LOVE: What are the implications of the ECOG-E2100 trial
comparing paclitaxel to paclitaxel plus bevacizumab? DR LOVE: What are the implications of the ECOG-E2100 trial
comparing paclitaxel to paclitaxel plus bevacizumab? |
 DR SEIDMAN: ECOG-E2100 was a pivotal study. This is the kind of information
you take home and interject into your practice the very next week.
Here, we’re looking at targeted therapy, where one doesn’t necessarily need
to test for the target. Docs who have been treating patients with metastatic
breast cancer already are well aware of using targeted therapy in the form
of trastuzumab. DR SEIDMAN: ECOG-E2100 was a pivotal study. This is the kind of information
you take home and interject into your practice the very next week.
Here, we’re looking at targeted therapy, where one doesn’t necessarily need
to test for the target. Docs who have been treating patients with metastatic
breast cancer already are well aware of using targeted therapy in the form
of trastuzumab.
In a sense, we now have a new targeted, better-tolerated, rationally designed
biologic therapy that doesn’t require testing of the tumor. I believe the design
of E2100 (3.1) was an appropriate one, using weekly paclitaxel as the control
arm — a regimen very close to my heart on the heels of CALGB-9840 — in
which we showed the superiority of weekly paclitaxel in terms of causing a
higher response rate and a longer time to progression than conventional paclitaxel
(Seidman 2004;[3.2]).
ECOG-E2100 showed us that the addition of bevacizumab to weekly paclitaxel
not only improved the response rate (Miller 2005a), as was observed in
the prior study with capecitabine (Miller 2005b), but unlike the study with
capecitabine, now we had an increase in time to progression of about five
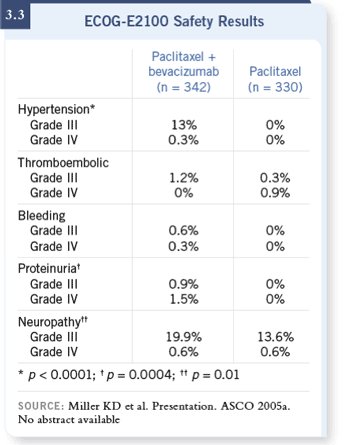
months (3.2). The additional costs in terms of toxicity were fairly modest,
with some increase in hypertension and proteinuria (Miller 2005a; [3.3]). Also,
a slight increase in the risk of Grade III neuropathy was seen with bevacizumab
(Miller 2005a; [3.3]).
 Track 3 Track 3
 DR LOVE: What’s your
take on the clinical implications
of ECOG-E2100
in terms of daily practice? DR LOVE: What’s your
take on the clinical implications
of ECOG-E2100
in terms of daily practice? |
  DR SEIDMAN: It has already
made an impact on our
daily practice at Memorial
Sloan-Kettering. I believe
the data speak very loudly for
themselves. This is the kind
of data where one doesn’t
go back and say, “Well,
I’m looking forward to six
months from now when the
FDA approves this.” DR SEIDMAN: It has already
made an impact on our
daily practice at Memorial
Sloan-Kettering. I believe
the data speak very loudly for
themselves. This is the kind
of data where one doesn’t
go back and say, “Well,
I’m looking forward to six
months from now when the
FDA approves this.”
This is the kind of information
where we feel almost an
ethical imperative to come
back and integrate it into
practice, the benefit being so
substantial.
For me, it’s a no-brainer for a patient who would appropriately receive a
taxane as monotherapy as first-line treatment of metastatic disease. That
woman should also receive bevacizumab, provided she has no serious contraindications
— brain metastases, uncontrolled hypertension or significant
proteinuria.
 DR LOVE: You mentioned using bevacizumab with taxane monotherapy.
Does that include docetaxel and nanoparticle albumin-bound (nab) paclitaxel? DR LOVE: You mentioned using bevacizumab with taxane monotherapy.
Does that include docetaxel and nanoparticle albumin-bound (nab) paclitaxel?
 DR SEIDMAN: The data that exist to date are with paclitaxel. We have a
history, though, that tells us, for example, with the integration of trastuzumab,
that with few exceptions, what worked with trastuzumab and paclitaxel also
worked with trastuzumab and docetaxel. DR SEIDMAN: The data that exist to date are with paclitaxel. We have a
history, though, that tells us, for example, with the integration of trastuzumab,
that with few exceptions, what worked with trastuzumab and paclitaxel also
worked with trastuzumab and docetaxel.
So do we really need to repeat every single experiment — for example, the
use of bevacizumab with weekly docetaxel, since it worked with paclitaxel
I think probably not. I would rather not see the reproduction of all of these
same Phase III studies over again with docetaxel that were done with paclitaxel.
I would ditto the same sentiment for nab paclitaxel with bevacizumab.
 DR LOVE: Would you want to see some Phase II or safety data with docetaxel
or nab paclitaxel combined with bevacizumab? DR LOVE: Would you want to see some Phase II or safety data with docetaxel
or nab paclitaxel combined with bevacizumab?
 DR SEIDMAN: Yes, I clearly want to see some safety data first. We’ve learned
to expect the unexpected. There certainly could be differences in patterns
of toxicity, based simply on the vehicles of those agents and how they might
interact with bevacizumab. DR SEIDMAN: Yes, I clearly want to see some safety data first. We’ve learned
to expect the unexpected. There certainly could be differences in patterns
of toxicity, based simply on the vehicles of those agents and how they might
interact with bevacizumab.
At Memorial, we’re about to embark on a trial in which we will be comparing
every three-week, every two-week and weekly dosing of nab paclitaxel with
bevacizumab as first-line therapy and also trastuzumab for patients with
HER2-positive disease. We’re looking at a nab paclitaxel schedule question
and also the safety of the co-administration of bevacizumab.
 Track 5 Track 5
 DR LOVE: Would you continue bevacizumab upon disease progression? DR LOVE: Would you continue bevacizumab upon disease progression? |
 DR SEIDMAN: I hope we don’t repeat the same mistake I believe we made
in the development of trastuzumab — to not definitively ask the research
question: “Is this an agent I should continue beyond the point of disease
progression?” DR SEIDMAN: I hope we don’t repeat the same mistake I believe we made
in the development of trastuzumab — to not definitively ask the research
question: “Is this an agent I should continue beyond the point of disease
progression?”
Although it was talked about for many years and it was attempted to determine
whether trastuzumab might be valuable to use beyond progression, we
simply don’t know the answer to that question on a clinical basis. Most of us
have developed a bias to continue trastuzumab for all practical purposes.
I believe that with bevacizumab, we have an opportunity to learn from the
lessons of the past and to design trials early on that look at the duration of
therapy question. If your patient progresses after her first-line taxane/bevacizumab
regimen, should you continue bevacizumab for the next course or the
course after and combine it with agents such as vinorelbine, gemcitabine and
capecitabine? I honestly don’t know the answer to that question.
 DR LOVE: Are there active discussions right now in the cooperative groups
about a trial like that, looking at bevacizumab or not on progression? DR LOVE: Are there active discussions right now in the cooperative groups
about a trial like that, looking at bevacizumab or not on progression?
 DR SEIDMAN: There are discussions, and in thinking about this question,
one of the reasons such trials have been thought, perhaps, not to be feasible
relates to the half-life of the antibody and the need for a wash-out period. The
argument with trastuzumab is that if you randomly assign patients to a do-notcontinue-
trastuzumab arm, pharmacologically, they’re still getting trastuzumab
many weeks after the last dose. DR SEIDMAN: There are discussions, and in thinking about this question,
one of the reasons such trials have been thought, perhaps, not to be feasible
relates to the half-life of the antibody and the need for a wash-out period. The
argument with trastuzumab is that if you randomly assign patients to a do-notcontinue-
trastuzumab arm, pharmacologically, they’re still getting trastuzumab
many weeks after the last dose.
There is no really good way around that issue, but I still believe it’s a clinically
relevant question to ask: Does stopping the antibody, as opposed to continuing
it with the next regimen, make a difference? If there is a pharmacologic effect,
it may translate early on into no difference at the first follow-up or the first set
of scans. But presumably, if continuing the antibody — in this case bevacizumab
— makes a difference and it’s a big enough difference, you may see that even regardless of the fact that the antibody will hang around for many weeks
after you discontinue it.
 Track 6 - 7 Track 6 - 7
 DR LOVE: Can you discuss what we know about nab paclitaxel? DR LOVE: Can you discuss what we know about nab paclitaxel? |
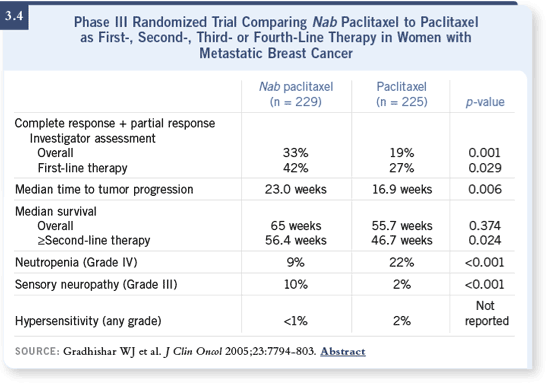
 DR SEIDMAN: Nab paclitaxel basically is paclitaxel formulated without
Cremophor. It was tested in the clinic in a large randomized trial involving
about 450 women (Gradishar 2005), and there does seem to be a differential
effect apart from the obvious advantage of not having to use premedications
to avoid the hypersensitivity reactions — no need for corticosteroids or
antihistamines (3.4). DR SEIDMAN: Nab paclitaxel basically is paclitaxel formulated without
Cremophor. It was tested in the clinic in a large randomized trial involving
about 450 women (Gradishar 2005), and there does seem to be a differential
effect apart from the obvious advantage of not having to use premedications
to avoid the hypersensitivity reactions — no need for corticosteroids or
antihistamines (3.4).
The administration of 260 mg/m2 nab paclitaxel caused a higher response rate
and a longer time to progression than the conventional 175 mg/m2 three-hour
infusion, every three-week regimen of Cremophor-based paclitaxel (Gradishar
2005; [3.4]). In my mind, this represents a real step forward.
There was less Grade III and IV neutropenia with 260 mg/m2 of nab paclitaxel
compared to 175 mg/m2 of paclitaxel (3.4), although this really didn’t translate
into a difference in febrile neutropenia, which was low in both arms. There
was a 10 percent incidence of Grade III neuropathy with nab paclitaxel, versus
a two percent incidence with Cremophor-based paclitaxel (Gradishar 2005;
[3.4]). This may not be all that surprising, given the differences in the doses
that were used.
There was an apparent, fairly rapid reversibility of the Grade III neuropathy.
The 10 percent of the population who experienced that degree of neuropathy
seemed to have a median time to resolution back to baseline of about
three weeks (Gradishar 2005), perhaps almost by the next cycle. Reversibility
seemed to take longer in the patients randomly assigned to Cremophor-based
paclitaxel.
I’m very intrigued by the data from the US Oncology Group Joanne Blum has
reported. A weekly regimen of nab paclitaxel — administered in a population
of patients who are already taxane-exposed to either paclitaxel or docetaxel
and many, but not all, of whom have some pre-existing neuropathy — seemed
not to cause a lot of severe neuropathy, particularly the 100 mg/m2 three weeks-
on and one-week-off regimen (Blum 2004).
 Track 8 Track 8
 DR LOVE: How would you compare the efficacy of weekly nab paclitaxel
compared to weekly paclitaxel? DR LOVE: How would you compare the efficacy of weekly nab paclitaxel
compared to weekly paclitaxel? |
 DR SEIDMAN: That’s a good question. Right now, it’s really hard to do.
The US Oncology Group treated nice-sized populations with their weekly
regimen. There were about 70 patients at the 100 mg/m2 dose (Blum
2004), and at the other dose — the 125 mg/m2 dose — in the range of
about 100 patients (O’Shaughnessy 2004). Since that’s a nice sample size
in terms of Phase II data, what we can say is that weekly nab paclitaxel has
activity in patients with prior taxane exposure and even with prior recent
taxane exposure. DR SEIDMAN: That’s a good question. Right now, it’s really hard to do.
The US Oncology Group treated nice-sized populations with their weekly
regimen. There were about 70 patients at the 100 mg/m2 dose (Blum
2004), and at the other dose — the 125 mg/m2 dose — in the range of
about 100 patients (O’Shaughnessy 2004). Since that’s a nice sample size
in terms of Phase II data, what we can say is that weekly nab paclitaxel has
activity in patients with prior taxane exposure and even with prior recent
taxane exposure.
Most of the data supporting the use of weekly Cremophor-based paclitaxel are
not in the same population but are in women who haven’t had prior taxanes
with a well-described activity with that approach from Phase II and the Phase
III randomized trial (Seidman 2004).
The randomized Phase II trial with docetaxel, published in the Annals of
Oncology by Tabernero (Tabernero 2004), is too difficult to compare with
these data. My sense, and this is a real “guesstimation,” is that I wouldn’t
expect weekly nab paclitaxel to be inferior to docetaxel.
There clearly are no direct comparisons, and I’m not sure I think that the
appetite is very strong in the cooperative groups to do these kinds of trials. I
believe there are too many other interesting targeted agents that beg asking
questions rather than how nab paclitaxel weekly stacks up to weekly docetaxel.
 Track 10 Track 10
 DR LOVE: Based on the current research data, where do you see the role
of nab paclitaxel in a clinical setting? DR LOVE: Based on the current research data, where do you see the role
of nab paclitaxel in a clinical setting? |
 DR SEIDMAN: The ability to deliver drugs more safely offers a real potential
benefit. Even if the randomized trial, perhaps, didn’t show a higher
response rate or a modestly longer time to progression compared to paclitaxel
(O’Shaughnessy 2003), simply not having to premedicate and not having to
worry about serious allergic reactions, in my mind, would make nab paclitaxel
the obvious choice. DR SEIDMAN: The ability to deliver drugs more safely offers a real potential
benefit. Even if the randomized trial, perhaps, didn’t show a higher
response rate or a modestly longer time to progression compared to paclitaxel
(O’Shaughnessy 2003), simply not having to premedicate and not having to
worry about serious allergic reactions, in my mind, would make nab paclitaxel
the obvious choice.
The issue that comes around with the emergence of any new drug is always
cost. There is a cost differential right now, since Cremophor-based paclitaxel
is generically available. It’s not a trivial differential. Even at Memorial Sloan-
Kettering, this is a consideration for the pharmacy budget.
We have guidelines for the use of nab paclitaxel, and the guidelines tend to
reflect the data. In scenarios that are parallel to those where nab paclitaxel’s
efficacy was shown — in the first-line and second-line setting — when one
would use paclitaxel, then I’m very motivated to substitute nab paclitaxel.
 DR LOVE: Have you utilized nab paclitaxel in the adjuvant setting, particularly
in patients who may be having problems with Cremophor-based paclitaxel
or docetaxel? DR LOVE: Have you utilized nab paclitaxel in the adjuvant setting, particularly
in patients who may be having problems with Cremophor-based paclitaxel
or docetaxel?
 DR SEIDMAN: I have used nab paclitaxel a handful of times so far in the
adjuvant setting. The scenarios are all memorable — patients who have had
strong allergic histories to other agents. One scenario involved a woman with
bipolar disease who becomes manic with steroid exposure, to the point where
I spoke with her psychiatrist about the need to avoid corticosteroids. DR SEIDMAN: I have used nab paclitaxel a handful of times so far in the
adjuvant setting. The scenarios are all memorable — patients who have had
strong allergic histories to other agents. One scenario involved a woman with
bipolar disease who becomes manic with steroid exposure, to the point where
I spoke with her psychiatrist about the need to avoid corticosteroids.
With appropriate permission from our Pharmacy and Therapeutics committee,
I was granted the approval to use nab paclitaxel in the adjuvant setting. This
was a nice thing that allowed us to administer appropriate anthracycline and
taxane-based adjuvant therapy without having to use corticosteroids.
Select publications
|

