|

| Tracks 1-8 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Single-agent versus combination
chemotherapy in metastatic
disease |
| Track 3 |
Dose modification with
capecitabine and impact
on efficacy |
| Track 4 |
Front-line fulvestrant following
adjuvant aromatase
inhibitor therapy |
|
| Track 5 |
Selection of hormone therapy
for metastatic disease
following adjuvant tamoxifen |
| Track 6 |
Dose and schedule of
fulvestrant in clinical practice |
| Track 7 |
Strategy of combining fulvestrant
with an aromatase inhibitor |
| Track 8 |
Hormonal therapy for
premenopausal patients
with metastatic disease |
|
|
Select Excerpts from the Interview*
 Track 2 Track 2
 DR LOVE: How do you approach deciding between combination versus
single-agent chemotherapy for metastatic disease? DR LOVE: How do you approach deciding between combination versus
single-agent chemotherapy for metastatic disease? |
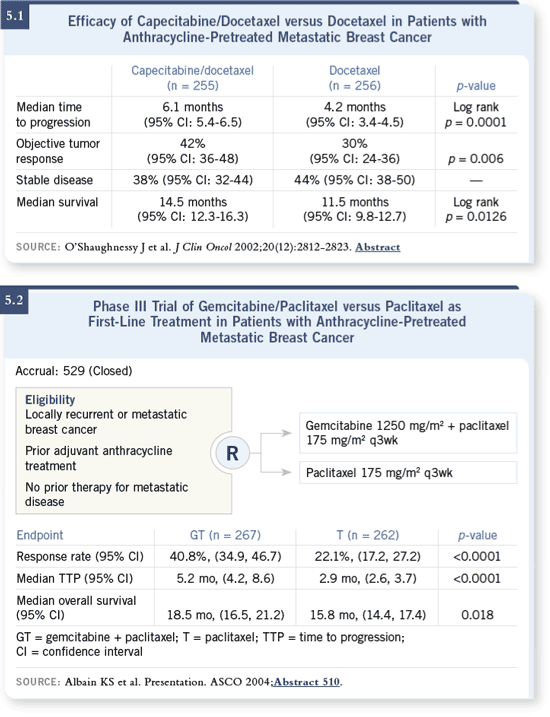
 DR VALERO: There are two combination regimens that have been proven to
be superior to single-agent taxane therapy. One is gemcitabine plus paclitaxel,
which was compared to paclitaxel alone. The data were presented at ASCO
last year, showing an improvement in time to progression and preliminary
evidence of an increase in overall survival (Albain 2004; [5.2]). DR VALERO: There are two combination regimens that have been proven to
be superior to single-agent taxane therapy. One is gemcitabine plus paclitaxel,
which was compared to paclitaxel alone. The data were presented at ASCO
last year, showing an improvement in time to progression and preliminary
evidence of an increase in overall survival (Albain 2004; [5.2]).
The other study compared docetaxel plus capecitabine to docetaxel alone
and also showed a time to progression and overall survival advantage
(O’Shaughnessy 2002; [5.1]).
Based on the evidence, both of these combinations are reasonable for firstline
chemotherapy of metastatic disease. However, in some patients, sequential
chemotherapy is our preference. I tend to use more sequential single-agent
chemotherapy, but I believe the role of combination chemotherapy in some
instances is well documented by the two studies I just mentioned.
 DR LOVE: Which situations, specifically? DR LOVE: Which situations, specifically?
 DR VALERO: For women who have symptomatic breast cancer with visceral
involvement, it is essential to have a response to alleviate the symptoms and
improve their quality of life. In those patients, in spite of the enhancement of
the adverse events, I strongly consider combination chemotherapy. DR VALERO: For women who have symptomatic breast cancer with visceral
involvement, it is essential to have a response to alleviate the symptoms and
improve their quality of life. In those patients, in spite of the enhancement of
the adverse events, I strongly consider combination chemotherapy.
 Track 3 Track 3
 DR LOVE: When you utilize capecitabine, what dose do you use? DR LOVE: When you utilize capecitabine, what dose do you use? |
 DR VALERO: In patients with a good performance status who are not heavily
pretreated, I use 2,000 mg/m2 daily in two divided doses for 14 of 21 days.
After two cycles of therapy, I will consider escalating the dose if the patient
has no toxicity. For patients with a poor performance status, in whom you’re
going to consider capecitabine as a second- or third-line therapy — patients
who are fragile — I may use 1,750 mg/m2 daily. DR VALERO: In patients with a good performance status who are not heavily
pretreated, I use 2,000 mg/m2 daily in two divided doses for 14 of 21 days.
After two cycles of therapy, I will consider escalating the dose if the patient
has no toxicity. For patients with a poor performance status, in whom you’re
going to consider capecitabine as a second- or third-line therapy — patients
who are fragile — I may use 1,750 mg/m2 daily.
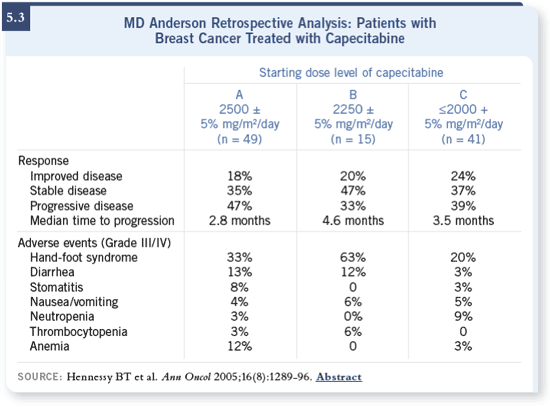
We recently published in the Annals of Oncology about our experience at MD
Anderson with different doses of capecitabine (Hennessy 2005; [5.3]). We
believe that a lower dose is preferable, even though the FDA-approved dose is
2,500 mg/m2.
I believe this publication really confirms what we do in the clinic. When
you have a Phase II study in several locations, but you select people out and
monitor them very closely, capecitabine can be administered at a higher dose. I
could deliver capecitabine at 2,500 mg/m2 daily, but it needs close monitoring
with a patient who is able to follow very closely with her oncologist. In the
clinical trials, as soon as the patients start to develop early signs of mucositis,
diarrhea or hand-foot syndrome, capecitabine is stopped immediately (5.4).
Then the patient reports to the oncologist or the research nurse for instructions.
Then you restart the capecitabine, and you may restart it at a lower dose.
So it needs some very close monitoring. In general, most patients at the end of
the day receive an average dose of around 2,000 mg/m2 daily as a single agent.
In some patients, I also use even lower doses — 1,500 mg/m2 daily.
The bottom line is — the evidence that a lower dose is efficacious is just
not there. Our study is one of the first that provides information (Hennessy
2005; [5.3]), but it was not a prospective study to assess response and time to
progression in a well-designed Phase II fashion.
 DR LOVE: Even though we don’t have definitive evidence right now, do you
feel that you’re compromising efficacy by reducing the dose? DR LOVE: Even though we don’t have definitive evidence right now, do you
feel that you’re compromising efficacy by reducing the dose?
 DR VALERO: I personally don’t. We have to remember that we are giving
palliative chemotherapy. I believe some of our traditional ways to develop
drugs in the metastatic setting haven’t been, in my opinion, optimal because
we escalate the dose until we make patients as sick as possible and then we
determine that’s the maximum tolerated dose (MTD). DR VALERO: I personally don’t. We have to remember that we are giving
palliative chemotherapy. I believe some of our traditional ways to develop
drugs in the metastatic setting haven’t been, in my opinion, optimal because
we escalate the dose until we make patients as sick as possible and then we
determine that’s the maximum tolerated dose (MTD).
Remember that MTD is based on administration of one cycle. Ideally, you
would like to develop a therapy in which you’re going to determine an MTD
for patients who receive at least four cycles, which is what you’re using in
the adjuvant setting. Most patients will receive a median of four cycles in the
metastatic setting.
 Track 4 - 5 Track 4 - 5
 DR LOVE: Can you overview what we know about fulvestrant, how you
utilize it in your practice, and how you dose it? DR LOVE: Can you overview what we know about fulvestrant, how you
utilize it in your practice, and how you dose it? |
 DR VALERO: I believe fulvestrant has been a major step in hormonal therapy
for women with estrogen or progesterone receptor-positive breast cancer. It
provides patients with an agent with a different mechanism of action. I have
been using it in many of my patients with hormone-sensitive breast cancer. DR VALERO: I believe fulvestrant has been a major step in hormonal therapy
for women with estrogen or progesterone receptor-positive breast cancer. It
provides patients with an agent with a different mechanism of action. I have
been using it in many of my patients with hormone-sensitive breast cancer.
 DR LOVE: What is your approach in the postmenopausal patient who’s had
prior adjuvant tamoxifen? DR LOVE: What is your approach in the postmenopausal patient who’s had
prior adjuvant tamoxifen?
 DR VALERO: Our approach in the institution is to use an aromatase inhibitor
up front and then fulvestrant as second-line therapy. Fulvestrant is approved
for patients who have failed tamoxifen, so you can use one agent or the other.
In the palliative setting, I believe you can use it either way. Fulvestrant has
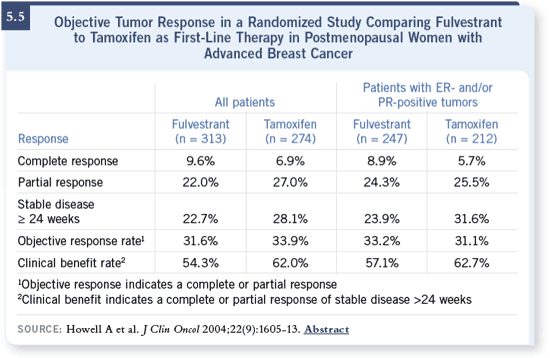
been shown to be as effective as anastrozole (Howell 2005, Robertson 2003)
and tamoxifen (Howell 2004; [5.5]). I use them in sequence. I don’t believe
there is any information that one sequence is better than the other. I use an
aromatase inhibitor, and then I use fulvestrant as a second-line therapy. DR VALERO: Our approach in the institution is to use an aromatase inhibitor
up front and then fulvestrant as second-line therapy. Fulvestrant is approved
for patients who have failed tamoxifen, so you can use one agent or the other.
In the palliative setting, I believe you can use it either way. Fulvestrant has
been shown to be as effective as anastrozole (Howell 2005, Robertson 2003)
and tamoxifen (Howell 2004; [5.5]). I use them in sequence. I don’t believe
there is any information that one sequence is better than the other. I use an
aromatase inhibitor, and then I use fulvestrant as a second-line therapy.
 DR LOVE: How do you dose and schedule fulvestrant? DR LOVE: How do you dose and schedule fulvestrant?
 DR VALERO: At MD Anderson, we use a loading dose. We administer 500
mg on day one and 250 mg on day 15 and day 29, and then monthly. Many of
the key investigators in the early development of the drug believe it is important to attain steady state. As you know, there is no randomized data for the
loading approach. DR VALERO: At MD Anderson, we use a loading dose. We administer 500
mg on day one and 250 mg on day 15 and day 29, and then monthly. Many of
the key investigators in the early development of the drug believe it is important to attain steady state. As you know, there is no randomized data for the
loading approach.
Currently, it is FDA approved at 250 mg monthly and is reimbursed by
Medicare at that dose. With all of those caveats, I believe — and I don’t know
if this is my bias — the loading approach is reasonable.
While we think that may be the best dosing schedule, we won’t know unless
we do a pharmacokinetic study and also, importantly, a very large study to
show that the doses are equally effective. I’m not sure if we’re going to be
seeing a dosing study large enough to determine efficacy. You can look at the
pharmacokinetics in a smaller study, but I don’t know if we’re going to see
efficacy differences.
 DR LOVE: Another potential strategy, which is being tested in the SoFEA
study (5.6), is combining an aromatase inhibitor with fulvestrant. Many oncologists
ask me about the patient who relapses on an aromatase inhibitor. Why
not keep her on the aromatase inhibitor and then add in fulvestrant? Is that
something you ever do or you think makes sense? DR LOVE: Another potential strategy, which is being tested in the SoFEA
study (5.6), is combining an aromatase inhibitor with fulvestrant. Many oncologists
ask me about the patient who relapses on an aromatase inhibitor. Why
not keep her on the aromatase inhibitor and then add in fulvestrant? Is that
something you ever do or you think makes sense?
 DR VALERO: You’re right on target. That is a huge controversy right now
about whether or not to maintain lower estrogen levels in a postmenopausal
patient and try a different hormonal manipulation. But we have to be cautious.
We learned from the ATAC study that lowering the estrogen levels and
bringing in something that competes for the ligand — in that case tamoxifen
— didn’t result in a benefit. If you look at most of the data with combined
hormonal therapy throughout a couple of decades, there really hasn’t been
clear evidence that two hormonal therapies are superior to one. DR VALERO: You’re right on target. That is a huge controversy right now
about whether or not to maintain lower estrogen levels in a postmenopausal
patient and try a different hormonal manipulation. But we have to be cautious.
We learned from the ATAC study that lowering the estrogen levels and
bringing in something that competes for the ligand — in that case tamoxifen
— didn’t result in a benefit. If you look at most of the data with combined
hormonal therapy throughout a couple of decades, there really hasn’t been
clear evidence that two hormonal therapies are superior to one.
 On the other hand, there is preclinical data generated by different laboratories
— including Kent Osborne’s lab — where lowering the ligand (in this
case estrogen) and using fulvestrant had a synergistic effect. This is the basis
of the randomized study of anastrozole plus or minus fulvestrant (SWOG-S0226;
[4.8]).
On the other hand, there is preclinical data generated by different laboratories
— including Kent Osborne’s lab — where lowering the ligand (in this
case estrogen) and using fulvestrant had a synergistic effect. This is the basis
of the randomized study of anastrozole plus or minus fulvestrant (SWOG-S0226;
[4.8]).
 DR LOVE: How do you approach hormonal therapy in a premenopausal
patient with metastatic disease? If the patient is on ovarian suppression,
would you consider fulvestrant or an aromatase inhibitor in that woman? DR LOVE: How do you approach hormonal therapy in a premenopausal
patient with metastatic disease? If the patient is on ovarian suppression,
would you consider fulvestrant or an aromatase inhibitor in that woman? |
 DR VALERO: We participated in the multicenter trial headed by Robert
Carlson from Stanford. In fact, we enrolled the majority of the patients on that
trial, which currently has 27 patients. It’s a Phase II study looking at hormonal
levels and the efficacy of goserelin and anastrozole (Carlson 2004; [5.7]). I’m
the principal investigator from MD Anderson. DR VALERO: We participated in the multicenter trial headed by Robert
Carlson from Stanford. In fact, we enrolled the majority of the patients on that
trial, which currently has 27 patients. It’s a Phase II study looking at hormonal
levels and the efficacy of goserelin and anastrozole (Carlson 2004; [5.7]). I’m
the principal investigator from MD Anderson.
With this minimal number of patients, we have been very impressed with the
combination, and the clinical benefit rate currently is 72 percent. I believe that
hormonal therapy in these women, which is the first biological therapy, makes
a lot of sense. Believe it or not, this is the only study that is currently available
to obtain preliminary efficacy data. The trial is still ongoing.
 What is the standard of care in patients who have failed tamoxifen and are
coming in for hormonal therapy? The standard of care is to use ovarian
ablation — medical or surgical. If the patient has had surgical ovarian ablation,
an aromatase inhibitor makes sense. Right now, I know that a lot of people are
using goserelin and an aromatase inhibitor.
What is the standard of care in patients who have failed tamoxifen and are
coming in for hormonal therapy? The standard of care is to use ovarian
ablation — medical or surgical. If the patient has had surgical ovarian ablation,
an aromatase inhibitor makes sense. Right now, I know that a lot of people are
using goserelin and an aromatase inhibitor.
Fulvestrant has not been studied a lot in patients who are premenopausal,
because there are not many premenopausal patients with ER-positive
disease who are coming in for a second hormonal therapy. But I believe
that is changing.
We’re using less cyclophosphamide, so fewer women are becoming menopausal
after receiving chemotherapy. More women who relapse may have ER-positive
disease and be premenopausal, and the pool may increase.
Select publications
|

