
 |
||||||||

| Tracks 1-21 | ||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 4, 5, 7
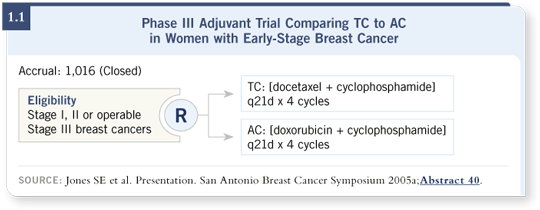
![]() DR JONES: We reported an adjuvant study in which we compared four cycles of standard-dose AC to four cycles of standard-dose TC (docetaxel and cyclophosphamide).
Chemotherapy was administered before radiation therapy or
tamoxifen, and we included patients with node-positive and higher-risk node-negative
disease ( Jones 2005a; [1.1]).
DR JONES: We reported an adjuvant study in which we compared four cycles of standard-dose AC to four cycles of standard-dose TC (docetaxel and cyclophosphamide).
Chemotherapy was administered before radiation therapy or
tamoxifen, and we included patients with node-positive and higher-risk node-negative
disease ( Jones 2005a; [1.1]).
When we started this trial in 1997, everyone was interested in combining doxorubicin with the taxanes, but we felt that we didn’t have enough data to combine docetaxel with doxorubicin. Consequently, we pursued this alternative route, which stands alone because it is one of the only nonanthracycline-containing regimens out there.
We now have mature results based on more than 170 events, with a median follow-up of 5.5 years. We have seen significantly fewer recurrences and events on the TC arm compared to the AC arm. I emphasized at San Antonio that the endpoint for this trial was disease-free survival, not overall survival. Overall survival was the secondary endpoint ( Jones 2005a).
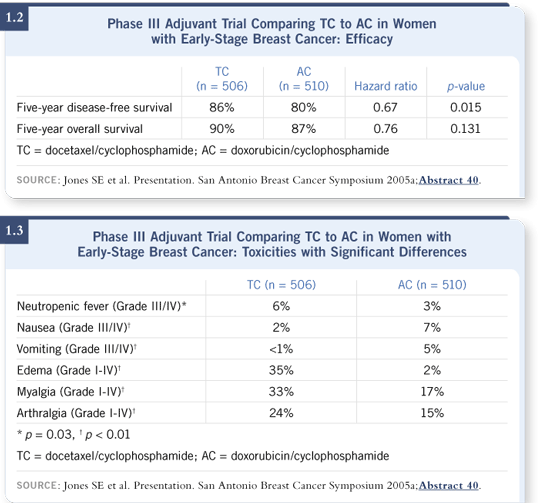
At five years, the disease-free survival was 86 percent for TC versus 80 percent for AC — a six percent absolute difference. The reduction in risk was roughly one third, and it was highly significant, with a p-value of 0.015. Also, a strong trend was favoring TC for overall survival — a three percent absolute difference at five years, with approximately a 24 percent reduction in the odds of dying from breast cancer ( Jones 2005a; [1.2]).
In general, TC was better tolerated. Some low-grade docetaxel-type side effects do occur, such as myalgias, arthralgias and edema, but they are fairly transient. The fever and neutropenia rates are also slightly higher; the numbers were 5.5 percent on the TC regimen and 2.5 percent on the AC regimen ( Jones 2005a; [1.3]). We didn’t use any prophylactic growth factors, but prophylactic antibiotics were used and encouraged.
The rate of CHF with AC was probably lower than would be expected. The usual figure that’s quoted is 0.5 to 1.0 percent; fortunately we haven’t seen that kind of rate. We have no reason to believe that TC would cause cardiac toxicity.
AC brought significantly more Grade III/IV nausea and vomiting, despite antiemetics ( Jones 2005a; [1.3]). That’s an unpleasant side effect we didn’t see with TC. I was amazed at how much better tolerated TC was than AC.
Track 9
![]() DR JONES: We are seeing some data that seem to break out on the basis of
ER status. If I present a patient with HER2-positive disease, it’s a no-brainer
that she needs trastuzumab. However, physicians haven’t generally been
thinking about patients with ER-positive disease requiring a different kind of
chemotherapy.
DR JONES: We are seeing some data that seem to break out on the basis of
ER status. If I present a patient with HER2-positive disease, it’s a no-brainer
that she needs trastuzumab. However, physicians haven’t generally been
thinking about patients with ER-positive disease requiring a different kind of
chemotherapy.
At the 2005 San Antonio Breast Cancer Conference, Cliff Hudis updated the dose-dense data and presented an unplanned exploratory analysis of the impact of ER status. Cliff ’s description of the results would be that there was less evidence of benefit among patients with ER-positive disease. My description would be that I didn’t see any evidence of benefit among patients with ER-positive disease.
Both of these viewpoints are probably a little extreme. However, almost all the treatment effect in their first study adding paclitaxel to AC (Henderson 2003) or in the update of the dose-dense trial (Hudis 2005) appears to be in the population with ER-negative disease. Yet now we have data that both TAC and TC appear to be equally effective in patients with ER-positive disease.
Another presentation at the 2005 San Antonio Breast Cancer Symposium, which followed mine, was the results from GEICAM 9906 by Professor Martin. The trial compared four cycles of FEC-90 followed by eight doses of weekly paclitaxel to six cycles of FEC-90; the paclitaxel arm received a slightly longer duration of therapy.
They saw an improvement in disease-free survival with the addition of weekly paclitaxel and a trend in overall survival. It appeared to be equally effective in patients with ER-positive versus ER-negative disease (Martin 2005). So we are seeing differences among some of these regimens.
Track 10
![]() DR JONES: We’ve actually just completed enrollment in our third US
Oncology adjuvant study, which compared AC followed by docetaxel every
three weeks to AC followed by capecitabine/docetaxel — the XT regimen.
Joyce O’Shaughnessy is the principal investigator of this trial, which tests XT
as part of an adjuvant regimen. We’ll have to see how that pans out.
DR JONES: We’ve actually just completed enrollment in our third US
Oncology adjuvant study, which compared AC followed by docetaxel every
three weeks to AC followed by capecitabine/docetaxel — the XT regimen.
Joyce O’Shaughnessy is the principal investigator of this trial, which tests XT
as part of an adjuvant regimen. We’ll have to see how that pans out.
In the context of practice, if you present to a patient the standard treatment as AC followed by docetaxel, and she decides not to enter a clinical trial, it’s hard to recommend something that you haven’t discussed with her. So many of us use AC followed by docetaxel.
![]() DR LOVE: Is that what you’re doing now that the study accrual is completed?
DR LOVE: Is that what you’re doing now that the study accrual is completed?
![]() DR JONES: We see many regimens used within US Oncology. Dose-dense
therapy is used for many patients. Some people are starting to use TC more.
DR JONES: We see many regimens used within US Oncology. Dose-dense
therapy is used for many patients. Some people are starting to use TC more.
We have a 30-year history with AC. It’s hard to say one day you should abandon it. I’ve heard people say that, but I’ve also heard the other extreme that they would only use TC in a patient with cardiac disease, in whom you wouldn’t want to take a chance with doxorubicin.
Track 11
![]() DR JONES: I believe they are relevant. In the manuscript we recently
submitted, it’s the one study I selected to compare because it was a larger study
with 3,000 women. Two thirds of the patients had node-negative disease and
one third had node-positive disease (Goldstein 2005).
DR JONES: I believe they are relevant. In the manuscript we recently
submitted, it’s the one study I selected to compare because it was a larger study
with 3,000 women. Two thirds of the patients had node-negative disease and
one third had node-positive disease (Goldstein 2005).
If we had the data when we started our trial in 1997, I believe we would have liked to combine doxorubicin and docetaxel, which is what they did, and compare it to AC. We weren’t comfortable enough to do that, so we used TC.
Their trial showed absolutely no difference in outcome, with about an 87 percent disease-free survival at four years in both arms. The curves were absolutely superimposable (Goldstein 2005). It’s hard to explain why TC was better than AC ( Jones 2005a) but AT was not (Goldstein 2005).
Part of the difference in the data sets could be that we used a higher dose of docetaxel — 75 mg/m2 ( Jones 2005a). In ECOG-E2197, they used docetaxel at 60 mg/m2 and doxorubicin at 60 mg/m2 (Goldstein 2005).
In metastatic breast cancer, a dose-response relationship exists with docetaxel, and Mouridsen will publish a paper evaluating different doses of docetaxel in metastatic disease. The numbers of patients aren’t huge, but clearly the lower dose of 60 mg/m2 is not quite as effective as 75 mg/m2, which isn’t quite as good as 100 mg/m2. Some of those differences aren’t statistically significant, but the trend is there (Mouridsen 2002).
Track 15
![]() DR JONES: We keep seeing a 16 to 17 percent rate of fever and neutropenia
with docetaxel as a single agent. Chuck Vogel conducted a study of docetaxel
with or without pegfilgrastim, and he showed a 17 percent rate of fever and
neutropenia for docetaxel alone versus one percent for docetaxel with pegfilgrastim
(Vogel 2005). I would probably use pegfilgrastim if I were going to
use docetaxel for many patients.
DR JONES: We keep seeing a 16 to 17 percent rate of fever and neutropenia
with docetaxel as a single agent. Chuck Vogel conducted a study of docetaxel
with or without pegfilgrastim, and he showed a 17 percent rate of fever and
neutropenia for docetaxel alone versus one percent for docetaxel with pegfilgrastim
(Vogel 2005). I would probably use pegfilgrastim if I were going to
use docetaxel for many patients.
In our AC versus TC study, we saw a higher rate of fever and neutropenia with TC. The actual numbers were 5.5 percent with TC, which is a far cry from 16 or 17 percent, and 2.5 percent with AC ( Jones 2005a; [1.3]). I see no justification for prophylactic white blood cell growth factors for regimens with a 2.5 to 3 percent incidence of fever and neutropenia. You might consider it for older patients or patients who have had severe, profound neutropenia even without fever during the first course.
![]() DR LOVE: When you use AC followed by docetaxel, do you use preemptive
growth factors?
DR LOVE: When you use AC followed by docetaxel, do you use preemptive
growth factors?
![]() DR JONES: Generally I don’t, but I’m starting to consider it. With the older
patients who have comorbidities, I believe we are close to having justification
for using growth factors.
DR JONES: Generally I don’t, but I’m starting to consider it. With the older
patients who have comorbidities, I believe we are close to having justification
for using growth factors.
Track 16
![]() DR JONES: We have piloted a trial of dose-dense AC followed by dose-dense
nanoparticle albumin-bound (nab) paclitaxel. Dose-dense nab paclitaxel is interesting
because you only need growth factors about one third of the time. Nick
Robert reported that in a pilot trial of about 30 patients (Robert 2005). That
would probably be our next study, but we have not come to a final decision.
DR JONES: We have piloted a trial of dose-dense AC followed by dose-dense
nanoparticle albumin-bound (nab) paclitaxel. Dose-dense nab paclitaxel is interesting
because you only need growth factors about one third of the time. Nick
Robert reported that in a pilot trial of about 30 patients (Robert 2005). That
would probably be our next study, but we have not come to a final decision.
We’re in the process of rethinking this at the moment. The world has changed with the introduction of bevacizumab. Suddenly, everyone wants to put bevacizumab into the adjuvant setting with the hope that it will be the next trastuzumab for patients with HER2-negative disease, which is 80 percent of the patients with breast cancer.
We’re also thinking about doing something further with the TC regimen. Up to this point, it stands alone. There are no other studies with TC, and no one is using it. The idea that immediately comes to mind is to combine TC with trastuzumab for patients with HER2-positive disease.
In BCIRG 006, docetaxel/carboplatin with trastuzumab wasn’t quite as good as an anthracycline-based regimen with trastuzumab. However, there was a separation of the disease-free survival curves, and Dr Slamon has been careful to point out that there was only a 20-event difference between the treatment groups (Slamon 2005).
Still, you wouldn’t rush to pick docetaxel/carboplatin/trastuzumab as your front-line regimen if it’s not quite as good. Dr Slamon has put forward the topoisomerase II (TOPO II) hypothesis to explain this, and it may be correct (Press 2005). We may end up with one treatment for the patients with nonamplified TOPO II and another treatment for those with amplified TOPO II.
For HER2-negative tumors, we now have a regimen up front — TC — that offers a significant improvement in disease-free survival compared to AC ( Jones 2005a; [1.2]), and we’ve not had that before. We also don’t have cardiac issues with TC, so you could think about combining it with trastuzumab, either concurrently or immediately after chemotherapy. We can’t go back and do a trial of TC with or without trastuzumab — that is no longer ethical — but we could look at concurrent or sequential therapy to obtain some toxicity data.
Track 19
![]() DR JONES: The bevacizumab story is interesting because, although I focus
on breast cancer, it seems to work in almost every tumor type in which it has
been studied. It is clearly active in breast cancer. If the study Kathy Miller
reported had been negative, I believe bevacizumab would have disappeared in
breast cancer, but it wasn’t negative (Miller 2005a, 2005b). It shows the same
order of benefit that we saw combining trastuzumab with chemotherapy in
metastatic disease, and suddenly everyone’s excited about moving bevacizumab
into the adjuvant setting.
DR JONES: The bevacizumab story is interesting because, although I focus
on breast cancer, it seems to work in almost every tumor type in which it has
been studied. It is clearly active in breast cancer. If the study Kathy Miller
reported had been negative, I believe bevacizumab would have disappeared in
breast cancer, but it wasn’t negative (Miller 2005a, 2005b). It shows the same
order of benefit that we saw combining trastuzumab with chemotherapy in
metastatic disease, and suddenly everyone’s excited about moving bevacizumab
into the adjuvant setting.
Studies are under way evaluating single agents — gemcitabine, docetaxel, doxorubicin, capecitabine and nab paclitaxel — with or without bevacizumab to prove efficacy. These are randomized studies; I believe it’s a 2:1 randomization favoring bevacizumab.
If the patient isn’t randomly assigned to receive bevacizumab, she can receive it at the time of progression. The idea is to try to obtain the same kind of data for the other active agents in breast cancer.
Track 20
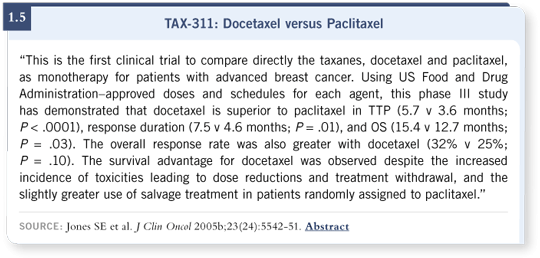
![]() DR JONES: I was the first author on the TAX-311 trial that was reported
in JCO in August 2005. In this study, we directly compared paclitaxel to
docetaxel at their FDA-approved doses and schedules — on an every three-week
basis. Docetaxel clearly showed more hematologic toxicity than paclitaxel,
but it showed a better response rate, time to tumor progression and
overall survival
DR JONES: I was the first author on the TAX-311 trial that was reported
in JCO in August 2005. In this study, we directly compared paclitaxel to
docetaxel at their FDA-approved doses and schedules — on an every three-week
basis. Docetaxel clearly showed more hematologic toxicity than paclitaxel,
but it showed a better response rate, time to tumor progression and
overall survival
(Jones 2005b; [1.5]).
In the last 12 years or so, however, weekly paclitaxel has been introduced. It probably falls somewhere in between, with less toxicity other than some neurotoxicity. I believe weekly paclitaxel has stepped up, and we see it in the adjuvant setting these days.
Track 21
![]() DR JONES: We’ve done a good deal of Phase II work with nab paclitaxel in metastatic breast cancer using the weekly schedules. Joanne Blum has been the
principal investigator for US Oncology on those trials (Blum 2004, 2003). It’s
an active regimen.
DR JONES: We’ve done a good deal of Phase II work with nab paclitaxel in metastatic breast cancer using the weekly schedules. Joanne Blum has been the
principal investigator for US Oncology on those trials (Blum 2004, 2003). It’s
an active regimen.
It does have some neurotoxicity, but it’s well tolerated. Also, you can
administer it in a short time. The novel formulation is what makes it really
intriguing — the way the drug is delivered. Other drugs could potentially be
administered this way also.
The lack of premedication is a big advantage. Some patients don’t do very well with steroid premedication, and they can’t sleep. That’s an issue for both paclitaxel and docetaxel. The lower amount of chair time with nab paclitaxel — depending on how busy you are — and the lack of need for special tubing can also be advantages.
![]() DR LOVE: Are you using nab paclitaxel in the clinical setting?
DR LOVE: Are you using nab paclitaxel in the clinical setting?
![]() DR JONES: We use it for selected patients — patients who may not have had
prior paclitaxel. The oncologists at US Oncology are probably more likely to
use it on our Phase II weekly schedule, which is very well tolerated.
DR JONES: We use it for selected patients — patients who may not have had
prior paclitaxel. The oncologists at US Oncology are probably more likely to
use it on our Phase II weekly schedule, which is very well tolerated.
![]() DR LOVE: How would you compare the neurotoxicity associated with nab paclitaxel to that associated with paclitaxel, particularly when using weekly
regimens for both?
DR LOVE: How would you compare the neurotoxicity associated with nab paclitaxel to that associated with paclitaxel, particularly when using weekly
regimens for both?
![]() DR JONES: Neurotoxicity is definitely associated with nab paclitaxel, but it
comes and goes very quickly. You do see neurotoxicity, but in two or three
weeks it resolves. In Nick Robert’s pilot study of dose-dense nab paclitaxel
(Robert 2005), some neurotoxicity required dose reductions, but it went away
pretty quickly. That is my clinical impression also.
DR JONES: Neurotoxicity is definitely associated with nab paclitaxel, but it
comes and goes very quickly. You do see neurotoxicity, but in two or three
weeks it resolves. In Nick Robert’s pilot study of dose-dense nab paclitaxel
(Robert 2005), some neurotoxicity required dose reductions, but it went away
pretty quickly. That is my clinical impression also.