
 |
||||||||

| Tracks 1-30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 5
![]() DR MILLER: The patients were treated with a single dose of bevacizumab and
then with docetaxel and doxorubicin added to bevacizumab for the rest of
their neoadjuvant therapy.
DR MILLER: The patients were treated with a single dose of bevacizumab and
then with docetaxel and doxorubicin added to bevacizumab for the rest of
their neoadjuvant therapy.
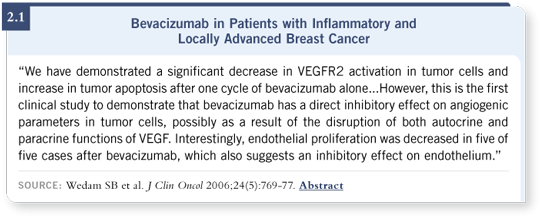
The investigators didn’t directly measure the tumor interstitial pressure, but they did use dynamic contrast-enhanced MRI to measure the perfusion and permeability of the vessels after an initial dose of bevacizumab and after a couple of doses of the combination therapy. They also measured the clinical response, and they collected blood and serum samples for several other circulating correlative studies (Wedam 2006; [2.1]).
What was perhaps most interesting was that they saw improvements in the appearance of the tumors of some of those patients with inflammatory breast cancer after their first dose of bevacizumab, before they’d even received chemotherapy. If you think about the intense vasculature and leakiness of those structures and the skin edema in inflammatory breast cancer, it’s not surprising to see improvement just with an anti-angiogenic agent, at least in the short term.
Track 9
![]() DR MILLER: The breast cancer program did not have any randomized dose-finding
studies. We conducted a sequential cohort study, initially planned to
evaluate two different dose levels — 3 mg/kg or 10 mg/kg every two weeks
(Cobleigh 2003). The 3-mg/kg dose was chosen because it was the dose from
the Phase I study that eliminated circulating VEGF, and the 10-mg/kg dose
was one of the highest doses that had been studied in the Phase I trial (Gordon
2001).
DR MILLER: The breast cancer program did not have any randomized dose-finding
studies. We conducted a sequential cohort study, initially planned to
evaluate two different dose levels — 3 mg/kg or 10 mg/kg every two weeks
(Cobleigh 2003). The 3-mg/kg dose was chosen because it was the dose from
the Phase I study that eliminated circulating VEGF, and the 10-mg/kg dose
was one of the highest doses that had been studied in the Phase I trial (Gordon
2001).
We thought 10 mg/kg showed greater activity than the 3-mg/kg dose. So the study was amended to evaluate an even higher dose of 20 mg/kg every two weeks. We didn’t see any increase in activity at 20 mg/kg, but we saw migraines, which was the dose-limiting toxicity that had not yet been identified (Cobleigh 2003).
The lung and colon programs proceeded a little differently in that they each did a randomized Phase II study. The colon trial compared 5 mg/kg to 10 mg/kg every two weeks (Kabbinavar 2003). The lung trial administered bevacizumab every three weeks but at doses equivalent to either 5 mg/kg or 2.5 mg/kg per week. In the lung cancer trial, the higher dose appeared to be superior (Johnson 2004), and in the colon cancer trial, the lower dose appeared to be superior (Kabbinavar 2003). So they used those doses going forward.
I believe this teaches us to be cautious with randomized Phase II studies, because they’re small and not designed to provide a direct comparison. Those results could easily have been spurious rather than teaching us anything true about doses. I don’t know that the dose we picked for the breast cancer trials is the right dose because it came from a fairly small, sequential Phase II study (Cobleigh 2003). So a randomized Phase III study is planned to evaluate two different doses of bevacizumab in breast cancer.
Tracks 13-15
![]() DR MILLER: The first one was the monotherapy Phase II trial that sequentially
enrolled 75 patients in three different dose cohorts. Overall, the objective
response rate was about nine percent, and about 17 percent of the patients
had disease that was responding or stable at five months, which is an unconventional
time point but one of the time points the protocol required to
evaluate the disease (Cobleigh 2003). So we know it’s a solid endpoint. Four
of those 75 patients were treated for at least a year without progression.
DR MILLER: The first one was the monotherapy Phase II trial that sequentially
enrolled 75 patients in three different dose cohorts. Overall, the objective
response rate was about nine percent, and about 17 percent of the patients
had disease that was responding or stable at five months, which is an unconventional
time point but one of the time points the protocol required to
evaluate the disease (Cobleigh 2003). So we know it’s a solid endpoint. Four
of those 75 patients were treated for at least a year without progression.
Those results led to a randomized Phase III trial for patients with refractory disease evaluating capecitabine alone or in combination with bevacizumab. This trial required patients to have received previous therapy with an anthracycline and a taxane; they could have received up to two previous chemotherapy regimens for metastatic disease. So they were a pretty advanced group (Miller 2005a).
The trial enrolled 462 patients and found essentially a doubling in the response rate by adding bevacizumab. The response rate went from nine percent to 19 percent in the eyes of the independent review facility and from about 19 percent to 30 percent in the eyes of the investigators. Those low response rates, however, did not budge the median progression-free survival, which was the primary endpoint, and it did not alter the overall survival (Miller 2005a; [2.2, 2.3]).
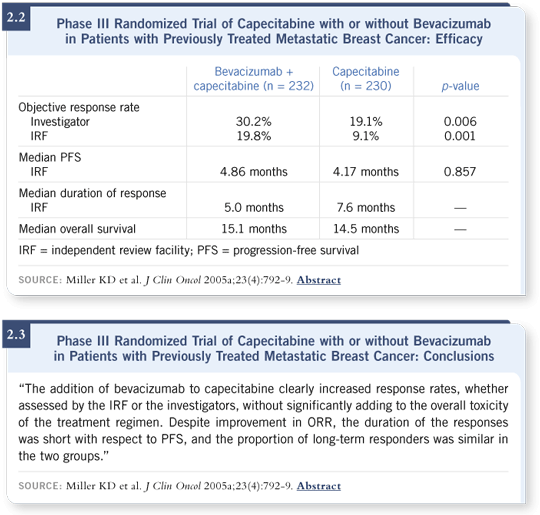
I reported it as a negative trial, although I was more encouraged than discouraged by the results. It was a difficult trial to report because the primary endpoint was progression-free survival. It had to be reported as a negative trial because it didn’t meet that endpoint. However, we learned a lot from that trial, and it brought more good news than bad news.
If you consider the correlative pathology studies showing that as breast cancers progress, the number of pro-angiogenic factors expressed increases, it’s hard to imagine how inhibiting one factor very late in the game would provide a demonstrable clinical effect or an effect that would last very long. However, inhibiting that same factor much earlier, when the system includes less redundancy, might provide a much greater effect.
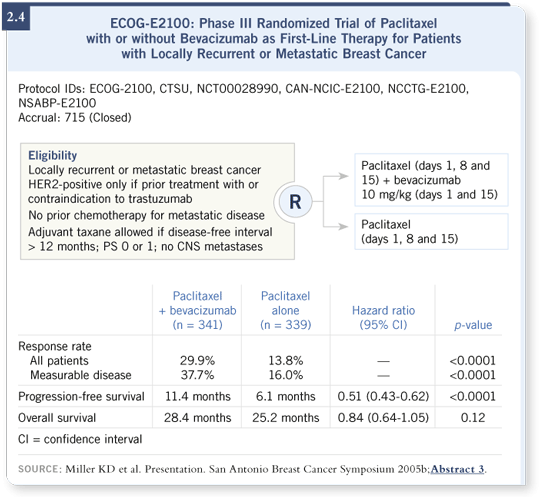
That was the idea behind ECOG-E2100, which had a similar design but used paclitaxel instead of capecitabine, primarily because we were looking at patients with earlier-stage disease. Most of the patients had not received a taxane as part of their adjuvant therapy, although about 18 percent of them had, and they had not received previous chemotherapy for recurrent disease. They were all randomly assigned to paclitaxel with or without bevacizumab (Miller 2005b; [2.4]).
ECOG-E2100 enrolled 680 eligible patients. In some ways, the results mirrored the earlier trial. It was essentially a doubling of response rates, although the baseline response rates were a bit higher than in the more refractory population. In this setting, that translated into a very striking improvement in progression-free survival of more than five months — from 6.1 months among the patients receiving paclitaxel alone to 11.4 months among the patients receiving the combination (Miller 2005b; [2.4]).
Tracks 17-19
![]() DR MILLER: ECOG-E2100 did not include a crossover. We made no provisions
for patients who were assigned to paclitaxel alone to receive bevacizumab
at the time of progression. For at least part of the duration of the trial, bevacizumab
was approved and commercially available for colon cancer. It’s possible
that a few of our patients might have had access to the drug for a crossover at
that point.
DR MILLER: ECOG-E2100 did not include a crossover. We made no provisions
for patients who were assigned to paclitaxel alone to receive bevacizumab
at the time of progression. For at least part of the duration of the trial, bevacizumab
was approved and commercially available for colon cancer. It’s possible
that a few of our patients might have had access to the drug for a crossover at
that point.
However, because bevacizumab was approved only for colon cancer and the costs are prohibitive for most patients, I believe the likelihood of crossover contaminating our study is minimal.
We talked about whether we should have a crossover in ECOG-E2100, and we decided not to for a couple of pragmatic reasons. One was that it would have made the trial a lot more complicated and expensive. Also, our primary endpoint was progression-free survival, so having a crossover would not have contributed to our primary endpoint.
At the time ECOG-E2100 was designed, we didn’t have the results from the bevacizumab/capecitabine trial (Miller 2005a), but we had those results within the first year of ECOG-E2100 being open for accrual.
Those patients who have progressed on their first chemotherapy regimen are the largest group of patients who enrolled in the bevacizumab/capecitabine trial, which found improvements in response rate but not in progression-free survival (Miller 2005a; [2.2]).
It was hard at that point to justify going back and amending ECOG-E2100 to include a crossover based on the results of a negative trial in that patient population.
Those who point to this as a criticism of the design and wonder if the survival data would be different if we had allowed for a crossover have a legitimate criticism. The data with more advanced disease suggest that it’s not likely to influence our results. Without a trial that includes a crossover, however, I don’t have data that will prove it.
![]() DR LOVE: Many look at the ECOG-E2100 results as a signal that has biologic
implications and, hopefully, implications for the adjuvant setting.
DR LOVE: Many look at the ECOG-E2100 results as a signal that has biologic
implications and, hopefully, implications for the adjuvant setting.
![]() DR MILLER: We believe the results will be even greater in the adjuvant
setting because first-line chemotherapy for metastatic disease is used fairly late
in the natural history of breast cancer. Although our patients hadn’t received
chemotherapy for metastatic disease, two thirds of them had received adjuvant
chemotherapy, and 18 percent had received a taxane (Miller 2005b).
DR MILLER: We believe the results will be even greater in the adjuvant
setting because first-line chemotherapy for metastatic disease is used fairly late
in the natural history of breast cancer. Although our patients hadn’t received
chemotherapy for metastatic disease, two thirds of them had received adjuvant
chemotherapy, and 18 percent had received a taxane (Miller 2005b).
These were not chemotherapy-naïve patients. They were much more advanced than the patients enrolled a decade ago in trials of first-line chemotherapy for metastatic disease. We expect much greater activity in the adjuvant setting, and recent laboratory data suggest that we’re likely to see it.
![]() DR LOVE: You mentioned the patients in ECOG-E2100 who had received
prior adjuvant taxanes. Can you talk about that?
DR LOVE: You mentioned the patients in ECOG-E2100 who had received
prior adjuvant taxanes. Can you talk about that?
![]() DR MILLER: In the design of ECOG-E2100, we allowed patients who had
received a taxane-containing adjuvant regimen to enroll as long as their
disease-free interval was at least 12 months. We did that for pragmatic reasons
because the taxanes were being used more frequently in the adjuvant setting.
We thought it would be reasonable to consider re-treating those patients if
their disease-free interval was at least a year.
DR MILLER: In the design of ECOG-E2100, we allowed patients who had
received a taxane-containing adjuvant regimen to enroll as long as their
disease-free interval was at least 12 months. We did that for pragmatic reasons
because the taxanes were being used more frequently in the adjuvant setting.
We thought it would be reasonable to consider re-treating those patients if
their disease-free interval was at least a year.
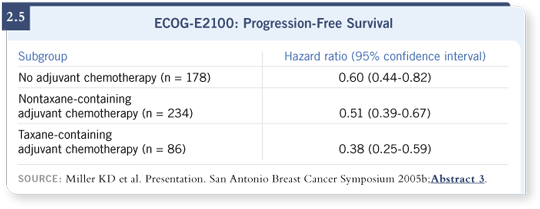
Approximately 18 percent of our patients had received a taxane-containing regimen. Their hazard ratio was 0.38 (Miller 2005b; [2.5]), which was the best hazard ratio of all of the clinically based subsets. For those patients, that translated into an improvement not from six to 11 months but from four to just more than 12 months in median progression-free survival (Miller 2005b).
Track 21
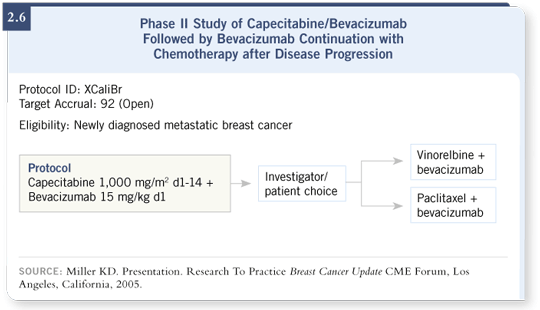
![]() DR MILLER: One of the trials that we activated shortly after we had the
results from ECOG-E2100 was a Phase II trial known as XCaliBr, which
uses the capecitabine/bevacizumab combination from the earlier Phase III
trial (Miller 2005a; [2.6]) but as first-line therapy for patients with metastatic
disease.
DR MILLER: One of the trials that we activated shortly after we had the
results from ECOG-E2100 was a Phase II trial known as XCaliBr, which
uses the capecitabine/bevacizumab combination from the earlier Phase III
trial (Miller 2005a; [2.6]) but as first-line therapy for patients with metastatic
disease.
It’s essentially the ECOG-E2100 patient population using the regimen from the capecitabine/bevacizumab trial (Miller 2005a). We thought that was a reasonable trial because we had ample safety data with the combination, and we knew that adding bevacizumab to capecitabine improved the response rates.
It potentially will provide patients in that first-line chemotherapy setting another option and one that would be oral and wouldn’t cause alopecia, if we see similar response rates and progression-free survival in a decent-sized Phase II study.
![]() DR LOVE: In colon cancer, bevacizumab adds to 5-FU, so you would expect
it to work.
DR LOVE: In colon cancer, bevacizumab adds to 5-FU, so you would expect
it to work.
![]() DR MILLER: You certainly would expect so. Our trial with refractory patients
found a doubling of response rates (Miller 2005a). We have data that strongly
suggest this would be active. What we don’t know is whether we’ll have the
same response rate and progression-free survival as with the paclitaxel-based
regimen. I believe that would be an important piece of data clinically to allow
people greater flexibility in their first-line regimen of chemotherapy with
bevacizumab.
DR MILLER: You certainly would expect so. Our trial with refractory patients
found a doubling of response rates (Miller 2005a). We have data that strongly
suggest this would be active. What we don’t know is whether we’ll have the
same response rate and progression-free survival as with the paclitaxel-based
regimen. I believe that would be an important piece of data clinically to allow
people greater flexibility in their first-line regimen of chemotherapy with
bevacizumab.
Tracks 22-23
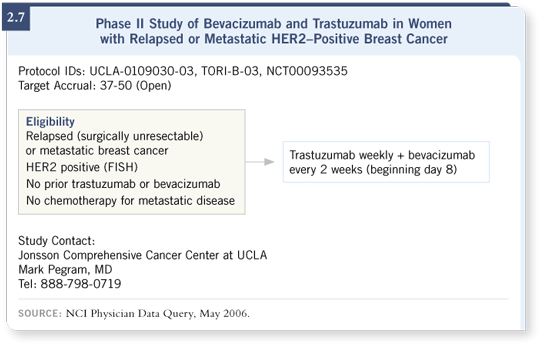
![]() DR MILLER: Mark Pegram and his group at UCLA are running a trial
(UCLA-0109030-03) combining bevacizumab with trastuzumab for patients
with HER2-positive disease (2.7). That’s a patient group for whom we
currently don’t have many clinical data with bevacizumab. They were excluded
from ECOG-E2100 or were required to have received trastuzumab previously,
but those patients are more likely to have increased VEGF expression.
DR MILLER: Mark Pegram and his group at UCLA are running a trial
(UCLA-0109030-03) combining bevacizumab with trastuzumab for patients
with HER2-positive disease (2.7). That’s a patient group for whom we
currently don’t have many clinical data with bevacizumab. They were excluded
from ECOG-E2100 or were required to have received trastuzumab previously,
but those patients are more likely to have increased VEGF expression.
That is certainly a population in which, based on the biology of their tumors, you would want to block both of those two signaling pathways. Dr Pegram’s Phase II trial will then be expanded, we hope, into a Phase III trial that has been proposed within ECOG and is currently being reviewed by the National Cancer Institute (NCI), which will evaluate a taxane/trastuzumab regimen with or without bevacizumab as first-line therapy for those patients.
![]() DR LOVE: Is that regimen eventually going to be evaluated in the adjuvant
setting?
DR LOVE: Is that regimen eventually going to be evaluated in the adjuvant
setting?
![]() DR MILLER: Discussions are ongoing within BCIRG about moving it into the
adjuvant setting in their next trial for patients with HER2-positive disease.
DR MILLER: Discussions are ongoing within BCIRG about moving it into the
adjuvant setting in their next trial for patients with HER2-positive disease.
I would also like more information about the patients with ER-positive disease who don’t yet need chemotherapy. The patients in ECOG-E2100 were receiving first-line chemotherapy for metastatic disease, but many of them had metastatic disease for several years and were treated sequentially with hormonal agents before enrolling in E2100. Estrogen increases VEGF expression, so a biologic rationale exists for combining bevacizumab with hormone-based therapies.
![]() DR LOVE: Are any trials evaluating that combination?
DR LOVE: Are any trials evaluating that combination?
![]() DR MILLER: A safety trial is ongoing with letrozole (UCSF-037518). It’s a
trial that I hope people will not look at in the wrong way and become disappointed.
I’ve already heard some people say they weren’t very impressed with
the response rates in the early reports.
DR MILLER: A safety trial is ongoing with letrozole (UCSF-037518). It’s a
trial that I hope people will not look at in the wrong way and become disappointed.
I’ve already heard some people say they weren’t very impressed with
the response rates in the early reports.
This trial was designed purely to look at safety. So it allowed patients who had been on an aromatase inhibitor for any period of time for metastatic disease, but whose disease was not actively progressing, to enroll and have bevacizumab added. Most of the patients reported so far had been on an aromatase inhibitor for quite some time before bevacizumab was added (Traina 2005).
I wouldn’t expect to see these patients, who had prolonged stable disease and didn’t have easily measurable disease, to suddenly show an easily identified objective response just by adding bevacizumab. It is definitely going to take a much larger study, with bevacizumab added at the time of the initial hormonal therapy, to really see the benefits.
However, this was a first step in investigating whether any unique safety issues arose from combining bevacizumab with hormone therapy. They certainly found no safety signals that would limit you from moving forward (Traina 2005).
Track 24
![]() DR MILLER: Some studies are evaluating bevacizumab in the neoadjuvant
setting. The one complicating factor is whether it will interfere with wound healing at the time of surgery. In Sandy Swain’s very small experience, five
patients had either wound dehiscence or significant seromas that seemed out
of proportion to what had been seen before, in both severity and chronicity of
those problems (Wedam 2006).
DR MILLER: Some studies are evaluating bevacizumab in the neoadjuvant
setting. The one complicating factor is whether it will interfere with wound healing at the time of surgery. In Sandy Swain’s very small experience, five
patients had either wound dehiscence or significant seromas that seemed out
of proportion to what had been seen before, in both severity and chronicity of
those problems (Wedam 2006).
This could be purely bad luck. These were all folks with very locally advanced disease. It’s a select population that makes its way to the NCI Clinical Center, but five out of 21 patients is a significant fraction, and that raises this as a concern in the neoadjuvant setting. We don’t know nearly enough to say that it’s prohibitive, and there’s certainly a lot that could be learned about biology in the neoadjuvant setting.
Track 25
![]() DR MILLER: The pilot adjuvant trial (ECOG-E2104; [2.8]) is enrolling
patients. That trial is critically important to us because it will evaluate adding
bevacizumab to an anthracycline-based treatment regimen. To date, approximately
100 patients in all have ever been treated with an anthracycline and
bevacizumab combined. All of those studies have raised some question of
increased cardiac toxicity.
DR MILLER: The pilot adjuvant trial (ECOG-E2104; [2.8]) is enrolling
patients. That trial is critically important to us because it will evaluate adding
bevacizumab to an anthracycline-based treatment regimen. To date, approximately
100 patients in all have ever been treated with an anthracycline and
bevacizumab combined. All of those studies have raised some question of
increased cardiac toxicity.
![]() DR LOVE: What’s the exact regimen in the pilot adjuvant trial?
DR LOVE: What’s the exact regimen in the pilot adjuvant trial?
![]() DR MILLER: The chemotherapy regimen in the pilot adjuvant trial is dose-dense
AC followed by paclitaxel, as used in CALGB-9741. ECOG-E2104
is observing two different cohorts. The first cohort receives bevacizumab
with the anthracycline and throughout therapy. The second cohort receives
bevacizumab only with paclitaxel, and this is our backup if we do see cardiac
toxicity issues with the combined administration (2.8). Hence, we’ll have
safety data with both strategies. The pilot adjuvant trial will enroll a total of
212 patients.
DR MILLER: The chemotherapy regimen in the pilot adjuvant trial is dose-dense
AC followed by paclitaxel, as used in CALGB-9741. ECOG-E2104
is observing two different cohorts. The first cohort receives bevacizumab
with the anthracycline and throughout therapy. The second cohort receives
bevacizumab only with paclitaxel, and this is our backup if we do see cardiac
toxicity issues with the combined administration (2.8). Hence, we’ll have
safety data with both strategies. The pilot adjuvant trial will enroll a total of
212 patients.
The full adjuvant trial will use a slightly different chemotherapy backbone that won’t require growth factors. We will be using AC on an every three-week basis followed by weekly paclitaxel. I wanted to use a weekly taxane regimen because the biggest support for moving this into the adjuvant setting is the data from ECOG-E2100, which used a weekly taxane schedule (Miller 2005b).
I don’t have direct data to say we wouldn’t have obtained the same improvements with an every three-week or every two-week taxane schedule, but the data we have are with a weekly schedule.
The full adjuvant trial has three arms, on which everybody receives the same chemotherapy. Patients in arm A receive no bevacizumab. Those in arm B receive six months of bevacizumab, concurrently with chemotherapy, and those in arm C receive 12 months of bevacizumab, six months with chemotherapy and an additional six months of maintenance.
The first six months of therapy are blinded and placebo controlled. At the end of the chemotherapy treatment, patients and their physicians will be told to which arm they have been assigned and whether they’re continuing bevacizumab for an additional six months.

Track 26
![]() DR MILLER: Adding bevacizumab increases the risk of arterial thrombotic
events, although to a very modest degree. We know a little about the risk
factors in that the risk seems to be preferentially borne out in patients who are
older than age 65 or those who have had previous arterial thrombotic events,
particularly MI, TIA or stroke.
DR MILLER: Adding bevacizumab increases the risk of arterial thrombotic
events, although to a very modest degree. We know a little about the risk
factors in that the risk seems to be preferentially borne out in patients who are
older than age 65 or those who have had previous arterial thrombotic events,
particularly MI, TIA or stroke.
This is not surprising. If you’re older or you’ve had one before, you’re at a greater risk of having such an event.
No reports associate cardiomyopathy or congestive heart failure with bevacizumab in any of the trials that either did not use concurrent anthracyclines or were in patient populations who would not have been previously treated with anthracyclines. So this is an issue specific to patients with breast cancer, sarcoma or leukemia, for which anthracyclines are used.
In the randomized bevacizumab/capecitabine trial, two patients had congestive heart failure or cardiomyopathy in the capecitabine-alone group compared to seven in the capecitabine with bevacizumab group (Miller 2005a). That sounds like an increase, but the overall event rate was so low that, statistically, those numbers were not different.
In ECOG-E2100, we didn’t see any sign of congestive heart failure when comparing the two groups (Miller 2005b). In Sandy Swain’s 21-patient experience, which is the only breast cancer trial that has used an anthracycline and bevacizumab concurrently, none of the patients had clinical congestive heart failure, but two of them showed a decrease in their ejection fraction to less than 40 percent (Wedam 2006).
Track 28
![]() DR MILLER: Aside from issues of cost and access to the drug, for patients
who would have been eligible for ECOG-E2100 — those receiving first-line
chemotherapy for metastatic disease who have not received an adjuvant taxane
within the last 12 months — I would strongly recommend treating them with
the E2100 regimen of weekly paclitaxel with bevacizumab.
DR MILLER: Aside from issues of cost and access to the drug, for patients
who would have been eligible for ECOG-E2100 — those receiving first-line
chemotherapy for metastatic disease who have not received an adjuvant taxane
within the last 12 months — I would strongly recommend treating them with
the E2100 regimen of weekly paclitaxel with bevacizumab.
No regimen has been shown to have the same improvements in progression-free survival with the lack of toxicity. Many patients are still being treated on ECOG-E2100 who are now out more than two years without progression. So I find those data compelling and hard to ignore.
The other thing that makes me say that so strongly is that we have data using bevacizumab for more refractory disease, and those patients don’t derive the same benefit. So, you can’t say, “We’re going to hold this in reserve. We’ll try something else first, and if this doesn’t work, then I’ll add bevacizumab.” That makes no more sense than saying the same thing about trastuzumab.
Yes, you can derive some benefit by using it later but not nearly the same amount as using it with first-line therapy.
![]() DR LOVE: What about the patients for whom you don’t want to use a taxane
because they may have diabetes or neuropathy?
DR LOVE: What about the patients for whom you don’t want to use a taxane
because they may have diabetes or neuropathy?
![]() DR MILLER: If someone otherwise meets the ECOG-E2100 criteria but has
some specific contraindication to a taxane, I would feel comfortable using one
of the regimens for which we have some safety and efficacy data. They include
the bevacizumab/capecitabine data from our previous randomized trial (Miller
2005a) and data with bevacizumab/vinorelbine from a Phase II trial conducted
at Dana-Farber (Burstein 2002).
DR MILLER: If someone otherwise meets the ECOG-E2100 criteria but has
some specific contraindication to a taxane, I would feel comfortable using one
of the regimens for which we have some safety and efficacy data. They include
the bevacizumab/capecitabine data from our previous randomized trial (Miller
2005a) and data with bevacizumab/vinorelbine from a Phase II trial conducted
at Dana-Farber (Burstein 2002).