|
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Can you bring us up to date on the new developments in
cancer and bone?
DR LOVE: Can you bring us up to date on the new developments in
cancer and bone?
![]() DR LIPTON: In terms of background and incidence, this year in the United
States 400,000 patients will be newly diagnosed with bone metastases. Most of
those will have breast or prostate cancer.
DR LIPTON: In terms of background and incidence, this year in the United
States 400,000 patients will be newly diagnosed with bone metastases. Most of
those will have breast or prostate cancer.
Three quarters of the patients who develop metastatic disease from breast or prostate cancer will have skeletal involvement. One third of the patients with lung cancer and approximately one third of the patients with kidney cancer who develop metastases will have skeletal involvement.
We also know from the placebo arms of the pamidronate and zoledronic acid trials that each patient who develops bone metastases will experience about one skeletal-related event (SRE) — defined as a bone fracture or the need for radiation or surgery to the bone — every three or four months without the use of a bisphosphonate. Skeletal involvement and SREs are terribly common in this patient population.
Track 5
![]() DR LOVE: Do we know how bisphosphonates have affected the clinical
course of metastatic cancer?
DR LOVE: Do we know how bisphosphonates have affected the clinical
course of metastatic cancer?
![]() DR LIPTON: These drugs have dramatically changed the face of metastatic
bone disease. In years gone by, it was not infrequent for patients to suffer
toward the end of their illness from hypercalcemia of malignancy.
DR LIPTON: These drugs have dramatically changed the face of metastatic
bone disease. In years gone by, it was not infrequent for patients to suffer
toward the end of their illness from hypercalcemia of malignancy.
We used to teach fellows and residents that 10 to 20 percent of patients with metastatic bone involvement would develop hypercalcemia, usually within three months of their demise. Now it’s pretty rare for a patient to develop hypercalcemia, and it’s extremely rare to see a patient in the hospital with hypercalcemia of malignancy.
Similarly, in the past it was not uncommon for a patient to be in the hospital with a pathologic fracture. We know that the rate of fracture has been significantly decreased by somewhere between 30 and 50 percent with the widespread use of the bisphosphonates. I believe we’ve dramatically changed the course of metastatic bone disease for patients, the complications thereof, the attendant costs, their performance status and their quality of life.
Track 6
![]() DR LOVE: What do we know about the side effects and complications
associated with the bisphosphonates?
DR LOVE: What do we know about the side effects and complications
associated with the bisphosphonates?
![]() DR LIPTON: They’re generally well tolerated compared to many chemotherapeutic
agents we routinely use. The major side effects seen with the bisphosphonates
can be categorized in two groups.
DR LIPTON: They’re generally well tolerated compared to many chemotherapeutic
agents we routinely use. The major side effects seen with the bisphosphonates
can be categorized in two groups.
The first group includes the acute phase reactions. Within 24 or 48 hours of administering an intravenous bisphosphonate, perhaps one third to one half of the patients will experience an increase in body temperature. This low-grade fever is usually self limiting and treated with acetaminophen.
A number of patients will experience exacerbation of bone pain. They’ll have arthralgias and increasing pain that is treated with narcotics. These symptoms usually diminish over 24 to 48 hours and are associated with the first dose of the bisphosphonate. With subsequent doses, you don’t see this as frequently.
Other long-term side effects are more serious. All bisphosphonates administered at a high enough dose over a shorter period of infusion will cause renal toxicity. They will cause glomerular or tubular damage, depending on which bisphosphonate is used. You need to monitor renal function every month before the bisphosphonate is administered.
Another complication, which has received a lot of press recently, is osteonecrosis of the jaw (ONJ). It was not previously recognized with oral bisphosphonates administered for metastatic breast cancer, although it does occur at a low rate with the oral bisphosphonates used for the treatment of osteoporosis.
ONJ was not recognized in the 3,000-patient Phase III studies of zoledronic acid or in the pamidronate database. It was recognized in 2003 by two oral surgeons — Bob Marx in Miami and Salvatore Ruggiero at Long Island Jewish Hospital. They both reported an influx of patients with ONJ in their oral surgery practices (Marx 2003; Ruggiero 2004).
Our knowledge about ONJ comes from two large retrospective studies. Cathy Van Poznak conducted the first one at Memorial Sloan-Kettering. Of approximately 900 patients treated with an intravenous bisphosphonate, she found an incidence of ONJ of 0.6 percent. The median duration of bisphosphonate therapy was about 52 months (Van Poznak 2004).
Dr Hoff at MD Anderson conducted a retrospective review of data from over 4,000 patients who received an intravenous bisphosphonate. The overall incidence of ONJ was 0.8 percent. It was about one percent for the patients with breast cancer, and it seemed to be slightly higher among the patients with multiple myeloma (Hoff 2006).
The outlier in terms of incidence of ONJ is from Professor Dimopoulis, who reported in the Greek experience an incidence of ONJ of around seven percent (Bamias 2005). In this country, the incidence is probably around one percent. It’s a phenomenon that is probably associated with bisphosphonate usage and appears to be more common the longer the drug is used.
Track 9
![]() DR LOVE: Where are we headed in the field of bone metastases?
DR LOVE: Where are we headed in the field of bone metastases?
![]() DR LIPTON: The treatment of bone metastases is in its relative infancy and has
been somewhat ignored, but I believe we’re headed into an exciting era. One
study by Ingo Diel and another by Trevor Powles and Sandy Paterson suggest
that adjuvant clodronate in addition to the usual adjuvant therapies can prevent
bone metastases and may prolong survival (Diel 1998; Powles 2006). One
negative study by Tiina Saarto using adjuvant clodronate showed the opposite
— a higher rate of metastasis (Saarto 2004) — but imbalances existed in
that study.
DR LIPTON: The treatment of bone metastases is in its relative infancy and has
been somewhat ignored, but I believe we’re headed into an exciting era. One
study by Ingo Diel and another by Trevor Powles and Sandy Paterson suggest
that adjuvant clodronate in addition to the usual adjuvant therapies can prevent
bone metastases and may prolong survival (Diel 1998; Powles 2006). One
negative study by Tiina Saarto using adjuvant clodronate showed the opposite
— a higher rate of metastasis (Saarto 2004) — but imbalances existed in
that study.
Adjuvant clodronate is being evaluated in NSABP-B-34. We’re waiting for events to occur to see if this large trial can confirm the two smaller studies by Diel and Powles. We hope that NSABP-B-34 will be reported in 2008.
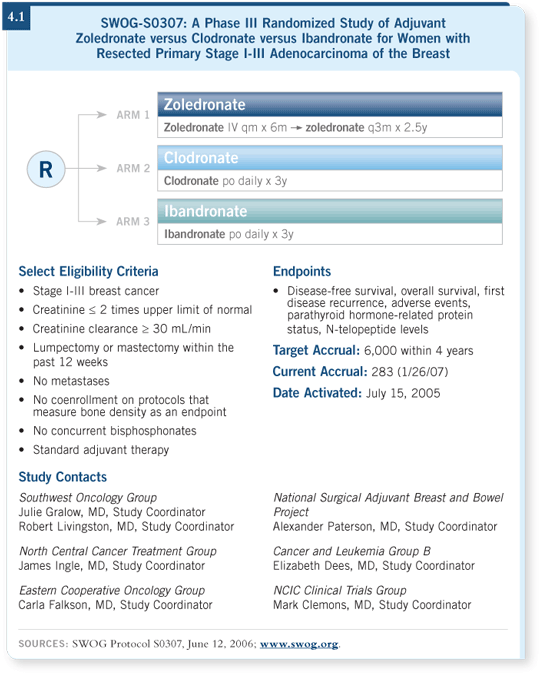
An Intergroup adjuvant study that will enroll 6,000 patients, being run by Julie Gralow, is comparing oral clodronate, oral ibandronate and intravenous zoledronic acid. It will be a comparison of two oral bisphosphonates and an intravenous bisphosphonate for the prevention of bone metastases (4.1). That study probably won’t be reported until 2010 or 2011.