
 |
||||||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you describe the objective of the NSABP-B-33 study and
what you reported at San Antonio (Mamounas 2006)?
DR LOVE: Can you describe the objective of the NSABP-B-33 study and
what you reported at San Antonio (Mamounas 2006)?
![]() DR MAMOUNAS: The B-33 trial was structured similarly to the NCIC-MA17
study (Goss 2003). Patients with ER-positive or PR-positive, Stage I or II
breast cancer who received tamoxifen for five years were randomly assigned to
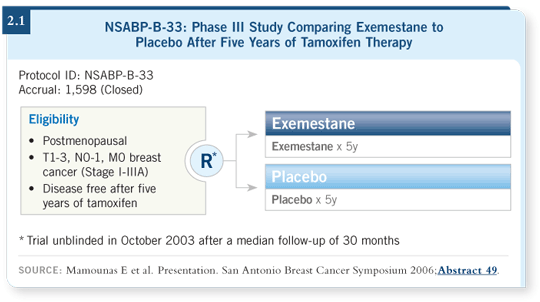
five years of exemestane or placebo (Mamounas 2006; [2.1]).
DR MAMOUNAS: The B-33 trial was structured similarly to the NCIC-MA17
study (Goss 2003). Patients with ER-positive or PR-positive, Stage I or II
breast cancer who received tamoxifen for five years were randomly assigned to
five years of exemestane or placebo (Mamounas 2006; [2.1]).
The projected sample size of the study was 3,000 patients. In October 2003, as the study was accruing, the results of the NCIC-MA17 trial indicating benefit with letrozole in that setting after five years of tamoxifen were disclosed. For ethical reasons, we stopped accruing and unblinded the trial, with the idea of offering exemestane to patients on placebo and allowing patients on exemestane to continue the drug if they chose. We did that in October of 2003, and at the time we had 1,598 patients.
Approximately half of the patients on placebo chose to receive exemestane, but it is unclear what happened to the other half of those patients. We didn’t collect that information, but it would be a mixture of alternatives: They may not have received any more therapy because they felt they were a long time from their diagnosis, or they may have received letrozole. Seventy-two percent of the patients who were on exemestane continued receiving exemestane. But the other 28 percent may not have received anything or may have received letrozole.
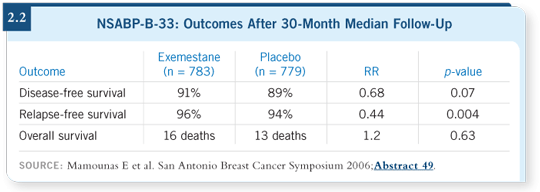
Like any other trial, the accrual started slowly and then ramped up. By the time we stopped, we were enrolling a lot of patients, but those patients did not contribute much to the overall data set. However, the fact that half of the patients were on exemestane and the other half were on placebo up to the point of unblinding apparently had an effect (2.2). On evaluation of the primary endpoint, a 32 percent reduction was evident in disease-free survival events. A 56 percent reduction was evident in relapse-free survival events, and this was statistically significant.
The median follow-up at that point was 30 months. Some reduction did occur in distant recurrence — about 31 percent — but this was not statistically significant. As in other studies, no survival difference appeared at this point, but very few deaths occurred: 16 in the exemestane group and 13 in the placebo group.
Interestingly, when we evaluated the subset analysis to see where the benefit was coming from, it was as we expected. Patients with node-positive disease at diagnosis, who had larger tumors, who had received prior chemotherapy or who were younger than age 60 — essentially the patients with more aggressive disease or disease with which you would expect events — were those who benefited the most from exemestane.
Track 6
![]() DR LOVE: Can you discuss the NSABP-B-42 trial, which addresses treatment
options after five years of adjuvant aromatase inhibitor therapy?
DR LOVE: Can you discuss the NSABP-B-42 trial, which addresses treatment
options after five years of adjuvant aromatase inhibitor therapy?
![]() DR MAMOUNAS: We realized that soon a large number of patients will be
reaching approximately five years of aromatase inhibitor therapy — or five
years of sequential therapy during which tamoxifen was administered for two
to three years and the aromatase inhibitor was administered for the remaining
two to three years.
DR MAMOUNAS: We realized that soon a large number of patients will be
reaching approximately five years of aromatase inhibitor therapy — or five
years of sequential therapy during which tamoxifen was administered for two
to three years and the aromatase inhibitor was administered for the remaining
two to three years.
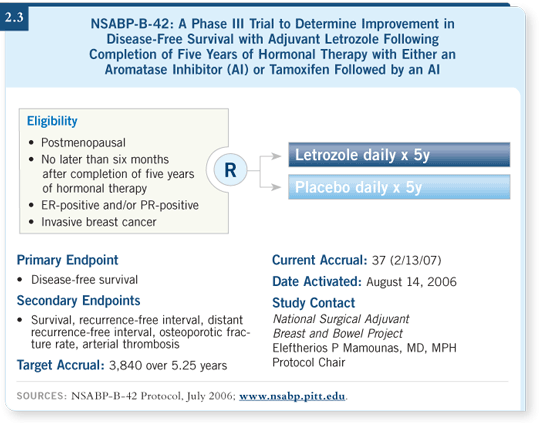
The B-42 trial is attempting to address the issue of duration for aromatase inhibitors after five years (2.3). It is similar to the B-14 trial, in which we administered five years of tamoxifen and then randomly assigned patients to an additional five years or not (Fisher 1996).
It’s by no means obvious that 10 years of an aromatase inhibitor will be better than five years. We believed that would be the case for tamoxifen, and we were wrong. When we unblinded the B-14 study, we found that the placebo group was a little superior to the tamoxifen group — almost statistically significant for disease-free survival. Whether this is applicable to aromatase inhibitors remains to be seen.
Jim Ingle presented data from the MA17 trial showing that if you receive letrozole for longer periods of time it’s better than if you receive it for shorter periods, but that was up to about four years (Ingle 2006). We don’t have any information on what will happen after five years with an aromatase inhibitor. We have to consider the risk-to-benefit ratio. It may be a little better, but at what price? How much osteoporosis and other side effects will we see in the long term?
Track 8
![]() DR LOVE: Can you discuss the design and rationale of the NSABPB-
40 study?
DR LOVE: Can you discuss the design and rationale of the NSABPB-
40 study?
![]() DR MAMOUNAS: As this trial was being developed, we were aware of the
ECOG-E2100 data with bevacizumab in the metastatic setting (Miller 2005).
We decided that B-40 would be a perfect setting in which to evaluate bevacizumab
as neoadjuvant therapy.
DR MAMOUNAS: As this trial was being developed, we were aware of the
ECOG-E2100 data with bevacizumab in the metastatic setting (Miller 2005).
We decided that B-40 would be a perfect setting in which to evaluate bevacizumab
as neoadjuvant therapy.
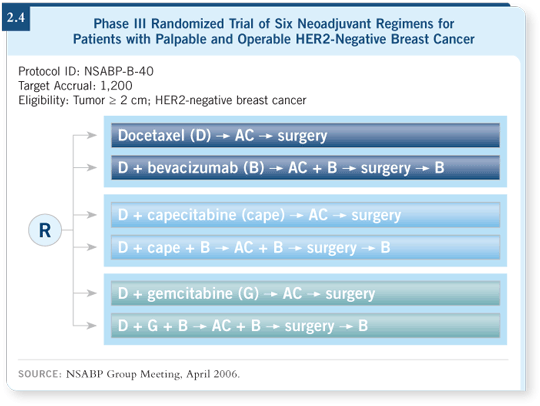
We wanted to administer bevacizumab with a taxane and AC-based regimen, but we had the issue of not wanting to administer bevacizumab right before surgery, given some of the wound-healing problems we have seen in previous trials.
As the trial currently stands, we start with either docetaxel ![]() AC or
docetaxel/capecitabine
AC or
docetaxel/capecitabine![]() AC or docetaxel/gemcitabine
AC or docetaxel/gemcitabine![]() AC. We administer
bevacizumab with all four cycles of the docetaxel-based regimen and with two
cycles of the AC and then allow a gap of six or eight weeks before the patient
goes to surgery (2.4).
AC. We administer
bevacizumab with all four cycles of the docetaxel-based regimen and with two
cycles of the AC and then allow a gap of six or eight weeks before the patient
goes to surgery (2.4).
After surgery, those patients randomly assigned to bevacizumab will continue on bevacizumab for a total of one year. This is to be on the same page as the future ECOG adjuvant trials, wherein one of the arms will receive at least one year of bevacizumab. The primary endpoint is pathologic complete response.
![]() DR LOVE: This study is only for patients with HER2-negative tumors?
DR LOVE: This study is only for patients with HER2-negative tumors?
![]() DR MAMOUNAS: Yes. As you know, we have now essentially separated the
treatment of those patients. In fact, we’ll be undertaking another trial in the
neoadjuvant setting for patients with HER2-positive disease.
DR MAMOUNAS: Yes. As you know, we have now essentially separated the
treatment of those patients. In fact, we’ll be undertaking another trial in the
neoadjuvant setting for patients with HER2-positive disease.
Track 12
![]() DR LOVE: What are your thoughts about the updated data from BCIRG
trial 006 presented at the San Antonio meeting?
DR LOVE: What are your thoughts about the updated data from BCIRG
trial 006 presented at the San Antonio meeting?
![]() DR MAMOUNAS: A big question has been the role of anthracyclines in the
treatment of HER2-positive breast cancer. Obviously, that became even more
controversial with the updated BCIRG 006 data indicating that the TCH
regimen performs essentially as well as AC
DR MAMOUNAS: A big question has been the role of anthracyclines in the
treatment of HER2-positive breast cancer. Obviously, that became even more
controversial with the updated BCIRG 006 data indicating that the TCH
regimen performs essentially as well as AC![]() TH (Slamon 2006).
TH (Slamon 2006).
![]() DR LOVE: Do these findings change your thoughts about the appropriate
therapy for patients with node-positive, HER2-positive disease?
DR LOVE: Do these findings change your thoughts about the appropriate
therapy for patients with node-positive, HER2-positive disease?
![]() DR MAMOUNAS: No, I believe the standard of therapy at this point is the FDA-approved
approach of administering an anthracycline followed by a taxane —
particularly paclitaxel — and trastuzumab, for a year. Obviously, with the new
data from BCIRG 006 (Slamon 2006), we’ll have to see what the role of TCH
is in this population of patients. That will evolve in the next few months.
DR MAMOUNAS: No, I believe the standard of therapy at this point is the FDA-approved
approach of administering an anthracycline followed by a taxane —
particularly paclitaxel — and trastuzumab, for a year. Obviously, with the new
data from BCIRG 006 (Slamon 2006), we’ll have to see what the role of TCH
is in this population of patients. That will evolve in the next few months.
![]() DR LOVE: In what kinds of situations would it be appropriate right now to
use adjuvant TCH or some other type of nonanthracycline regimen with
trastuzumab?
DR LOVE: In what kinds of situations would it be appropriate right now to
use adjuvant TCH or some other type of nonanthracycline regimen with
trastuzumab?
![]() DR MAMOUNAS: You can use it in pretty much all patients with HER2-
positive disease because it’s clearly a regimen with minimal cardiotoxicity.
Some depression in left ventricular ejection fraction (LVEF) occurred, but that
recovered after the discontinuation of trastuzumab. If you want to be more
selective, that would be a good regimen for any patient for whom cardiac
function is a concern.
DR MAMOUNAS: You can use it in pretty much all patients with HER2-
positive disease because it’s clearly a regimen with minimal cardiotoxicity.
Some depression in left ventricular ejection fraction (LVEF) occurred, but that
recovered after the discontinuation of trastuzumab. If you want to be more
selective, that would be a good regimen for any patient for whom cardiac
function is a concern.