You are here: Home: BCU 1| 2005: Grand Rounds
| |
|
|
| |

| 4.1 |
|
| |
|

SLIDE 4.1 All three major systemic treatment strategies for breast cancer have been evaluated in the neoadjuvant setting. This slide set focuses on a number of key clinical trials evaluating neoadjuvant chemotherapy, endocrine treatment and biologic therapy.
|
| |
|
|
| 4.2 |
|
| |
|
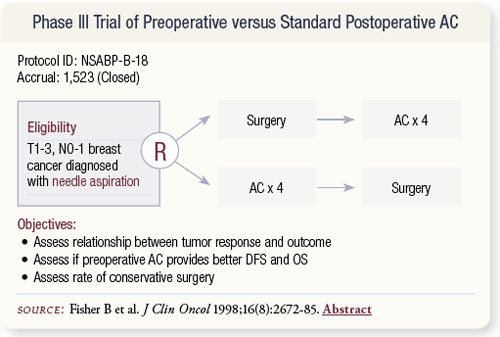
SLIDE 4.2 NSABP-B-18 was a classic randomized trial that enrolled over 1,500 women who had operable breast cancer between 1988 and 1993. Women were randomly assigned to either surgery followed by AC or AC followed by surgery.
|
| |
|
|
| 4.3 |
|
| |
|
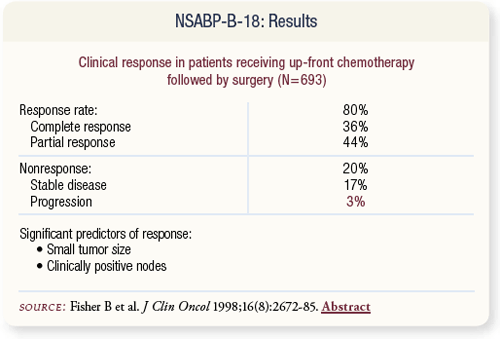
SLIDE 4.3 In women receiving preoperative chemotherapy, the inbreast response rate was 80 percent. Progression during chemotherapy was three percent.
|
| |
|
|
| 4.4 |
|
| |
|
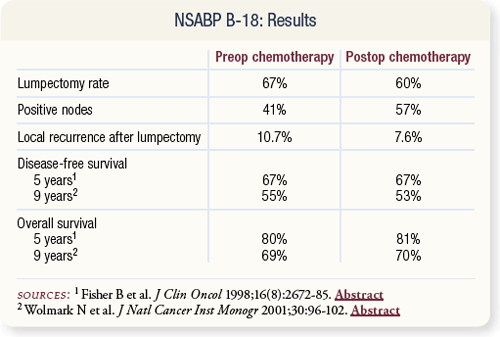
SLIDE 4.4 The lumpectomy rate was significantly higher in women who received preoperative therapy (67 percent) compared to those who had initial surgery (60 percent). No difference in disease-free or overall survival was evident for patients receiving preoperative versus postoperative chemotherapy.
|
| |
|
|
| 4.5 |
|
| |
|
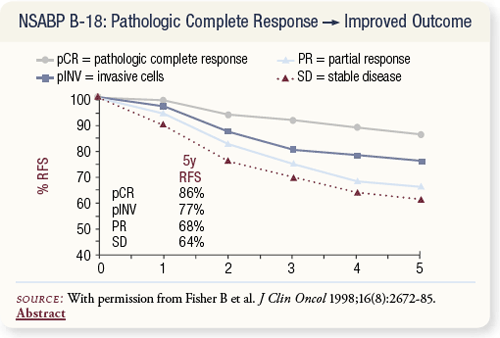
SLIDE 4.5 A striking correlation was noted between response in the breast and relapse-free survival. Women who had a pathologic complete response (pCR) were three times less likely to have a recurrence than women who had stable disease.
|
| |
|
|
| 4.6 |
|
| |
|
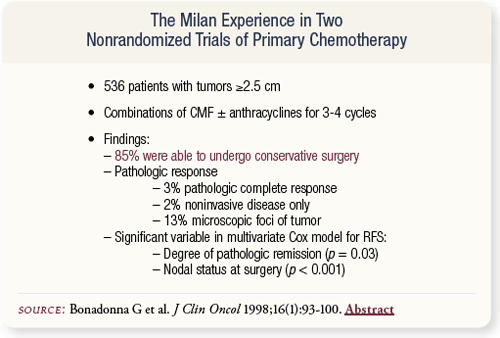
SLIDE 4.6 The experience from Milan with preoperative chemotherapy includes two sequential nonrandomized trials with over 500 patients. In patients who had tumors 2.5 centimeters or larger and were treated with preoperative therapy, 85 percent were able to undergo conservative surgery.
|
| |
|
|
| 4.7 |
|
| |
|
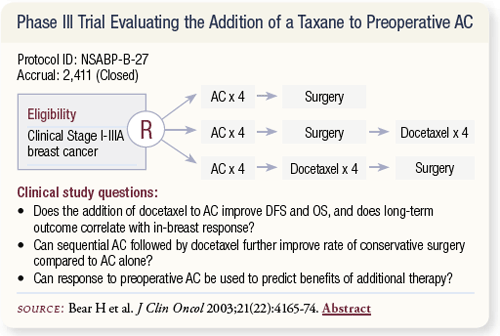
SLIDE 4.7 NSABP-B-27 closed in December 2000 after enrolling 2,411 patients. There were three randomized arms: AC alone followed by surgery; AC followed by surgery followed by four cycles of docetaxel; and AC followed by docetaxel followed by surgery.
|
| |
|
|
| 4.8 |
|
| |
|
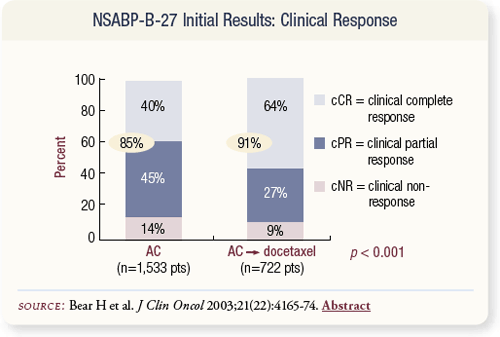
SLIDE 4.8 The data were first presented in San Antonio in 2001 and were subsequently published in 2003 in the Journal of Clinical Oncology. A statistically significant (85 percent versus 91 percent) difference occurred in the overall response rate in the breast.
|
| |
|
|
| 4.9 |
|
| |
|
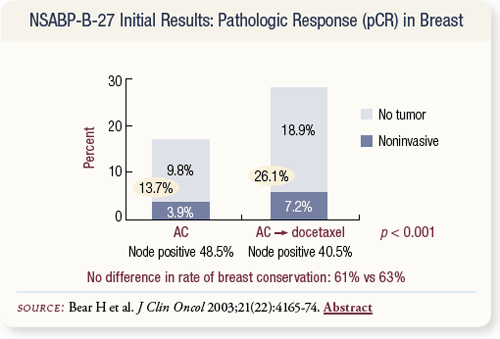
SLIDE 4.9 Pathologic complete response rate was nearly doubled. No difference was observed in the breast conservation rate.
|
| |
|
|
| 4.10 |
|
| |
|
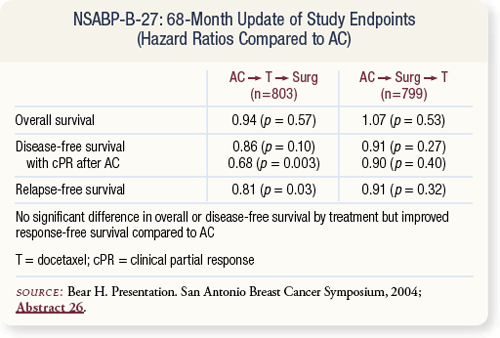
SLIDE 4.10 NSABP-B-27 reported 68-month follow-up at San Antonio in 2004. The addition of docetaxel did not result in a statistically significant improvement in overall or disease-free survival but did result in improved relapse-free survival.
|
| |
|
|
| 4.11 |
|
| |
|
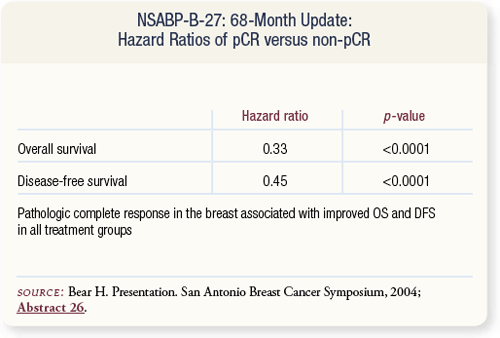
SLIDE 4.11 Achievement of pCR was associated with a highly significant improvement in overall and disease-free survival, regardless of which treatment regimen patients received.
|
| |
|
|
| 4.12 |
|
| |
|
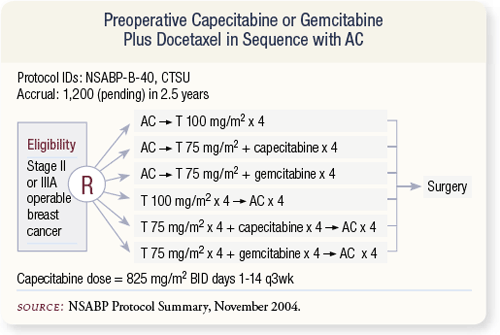
SLIDE 4.12 NSABP-B-40 will replace trial B-27 and is expected to open in early 2005. The target accrual is approximately 1,200 patients. Patients will be randomly assigned to one of six chemotherapy regimens evaluating preoperative capecitabine or gemcitabine plus docetaxel in sequence with AC.
|
| |
|
|
| 4.13 |
|
| |
|
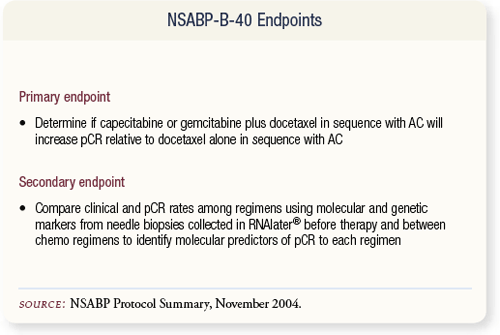
SLIDE 4.13 The primary objective of B-40 is to determine whether docetaxel-based combinations in sequence with AC will increase the pCR rate compared to docetaxel alone in sequence with AC. The NSABP will also attempt to identify genetic and molecular markers predictive of pCR to each chemotherapy regimen.
|
| |
|
|
| 4.14 |
|
| |
|
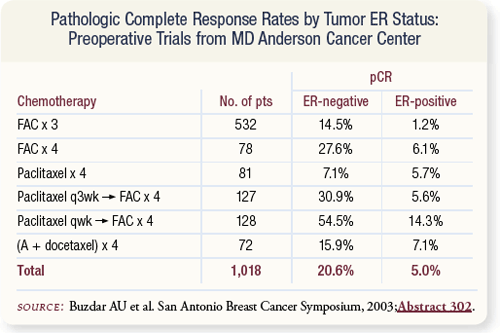
SLIDE 4.14 Aman Buzdar and colleagues evaluated a series of preoperative chemotherapy trials conducted at MD Anderson to correlate pCR with hormone receptor status. A 20 percent pCR rate occurred in women with ER-negative disease compared to five percent in women with ER-positive disease.
|
| |
|
|
| 4.15 |
|
| |
|
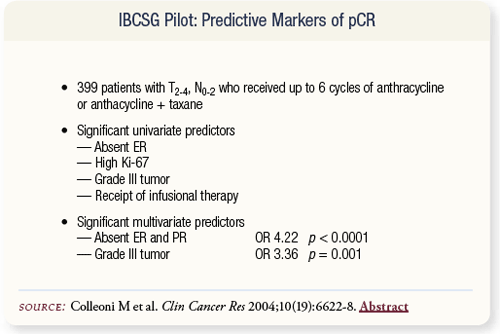
SLIDE 4.15 The International Breast Cancer Study Group enrolled 399 patients in a pilot trial evaluating factors predictive of pCR. The absence of estrogen and progesterone receptor status and high tumor grade were related to pCR.
|
| |
|
|
| 4.16 |
|
| |
|

SLIDE 4.16 Another neoadjuvant strategy is endocrine treatment, both in premenopausal and postmenopausal patients.
|
| |
|
|
| 4.17 |
|
| |
|
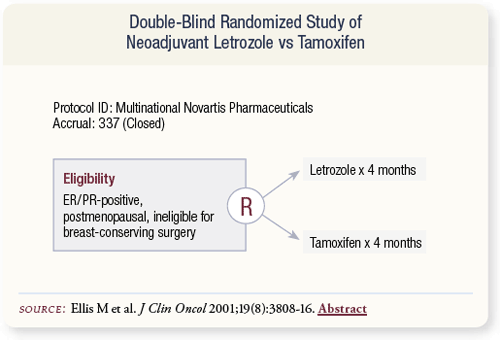
SLIDE 4.17 In one major study, 337 postmenopausal patients with primary breast cancer who were ineligible for breast-conserving surgery were randomly assigned to four months of letrozole or tamoxifen prior to re-evaluation for surgery.
|
| |
|
|
| 4.18 |
|
| |
|
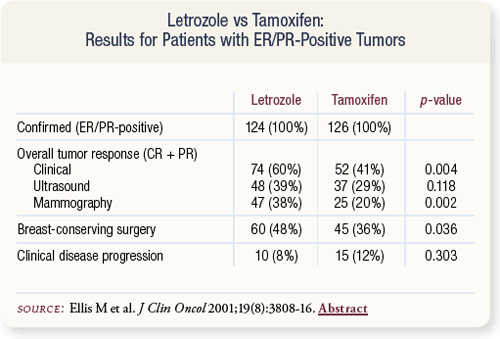
SLIDE 4.18 Patients treated with letrozole experienced greater tumor response than patients treated with tamoxifen. Conversion from ineligibility to breast-conserving surgery was more frequent in the letrozole arm (48 percent) than in the tamoxifen arm (36 percent).
|
| |
|
|
| 4.19 |
|
| |
|
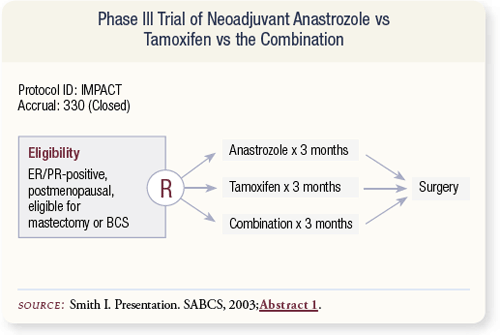
SLIDE 4.19 The IMPACT study presented by Ian Smith and Mitchell Dowsett in San Antonio in 2003 randomly assigned 330 patients to preoperative anastrozole, tamoxifen or the combination. Only 56 percent of patients were judged to require mastectomy prior to therapy.
|
| |
|
|
| 4.20 |
|
| |
|
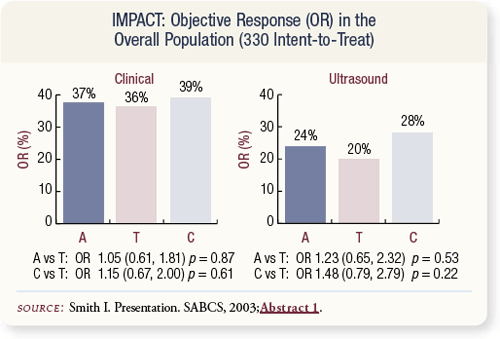
SLIDE 4.20 Clinical response rates were similar across the three arms. Note that all patients in the letrozole study were ineligible for breast-conserving surgery, whereas only 56 percent of patients in IMPACT required mastectomy at baseline. The smaller tumor size in IMPACT may have made clinical assessment more difficult.
|
| |
|
|
| 4.21 |
|
| |
|
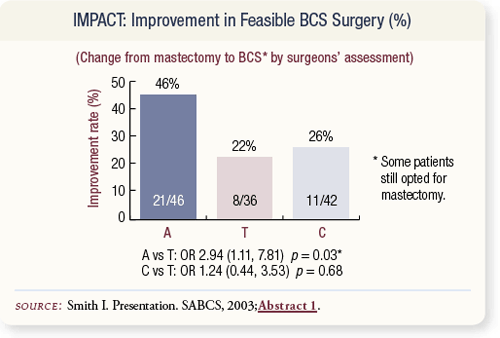
SLIDE 4.21 Of the patients initially judged to need a mastectomy, anastrozole resulted in a doubling of the eligibility for conservative surgery. Forty-six percent of those patients were able to undergo less than a mastectomy versus 22 and 26 percent of patients in the tamoxifen alone and combination arms, respectively.
|
| |
|
|
| 4.22 |
|
| |
|
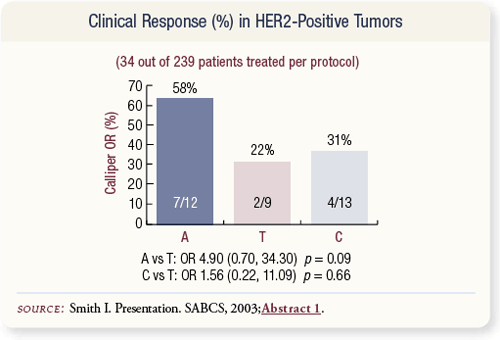
SLIDE 4.22 A relatively small percentage of patients had HER2- positive disease — 34 out of 239 patients. A trend was observed toward improved response rates with anastrozole (58 percent) compared to tamoxifen (22 percent).
|
| |
|
|
| 4.23 |
|
| |
|
SLIDE 4.23 Another systemic strategy being tested in the neoadjuvant setting is biologic therapy. To this point, the major available data has come from trials of neoadjuvant trastuzumab in patients with HER2-positive tumors.
|
| |
|
|
| 4.24 |
|
| |
|
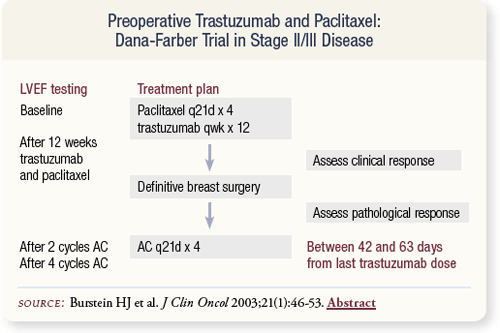
SLIDE 4.24 Dana-Farber conducted a series of studies evaluating trastuzumab in combination with chemotherapy. Harold Burstein and colleagues reported a study of preoperative trastuzumab and paclitaxel in the Journal of Clinical Oncology in 2003.
|
| |
|
|
| 4.25 |
|
| |
|
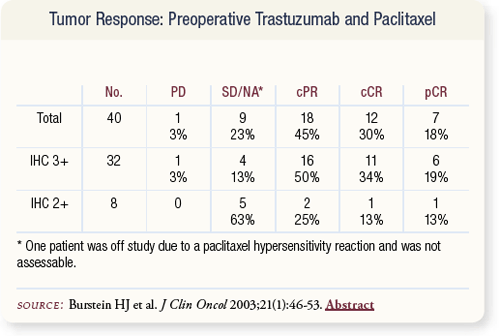
SLIDE 4.25 The overall pCR rate in this trial was 19 percent in patients whose tumors were IHC 3+ and 13 percent in patients with IHC 2+ scoring.
|
| |
|
|
| 4.26 |
|
| |
|
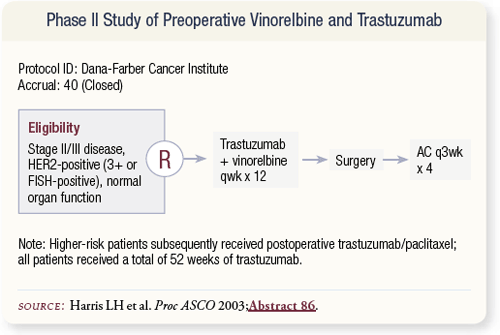
SLIDE 4.26 Lindsey Harris at Dana-Farber conducted a Phase II study evaluating trastuzumab and vinorelbine based on data with that combination in the metastatic setting. Approximately 60 percent of patients had Stage III disease.
|
| |
|
|
| 4.27 |
|
| |
|
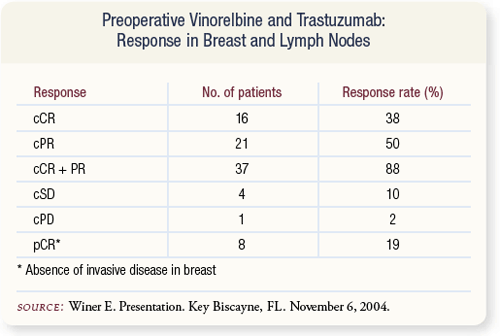
SLIDE 4.27 These data were initially presented at ASCO in 2003. The results are strikingly similar to their prior study of preoperative trastuzumab and paclitaxel. Overall response rate was 88 percent with pCR rate of 19 percent.
|
| |
|
|
| 4.28 |
|
| |
|
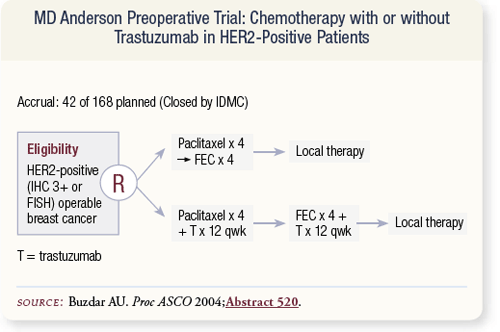
SLIDE 4.28 Aman Buzdar at ASCO 2004 presented findings from a trial in patients with HER2-positive operable breast cancer. The study compared four cycles of paclitaxel followed by four cycles of FEC compared to this same regimen with concurrent trastuzumab. Target accrual was 168 patients, but accrual to the trial was stopped by the MD Anderson Data Safety Monitoring Board.
|
| |
|
|
| 4.29 |
|
| |
|
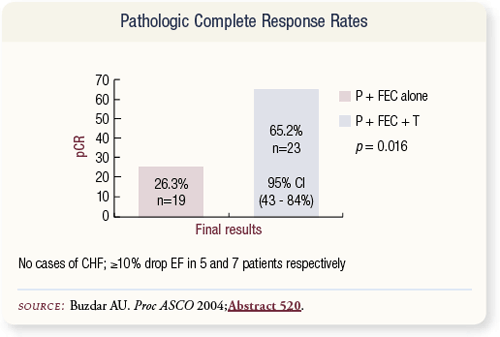
SLIDE 4.29 Patients who received concurrent preoperative chemotherapy and trastuzumab had a significantly higher pCR rate (65 percent) than patients who received chemotherapy alone (25 percent). The regimen appeared to be tolerable in spite of the administration of concurrent trastuzumab and epirubicin. No incidents of congestive heart failure were observed.
|
| |
|
|
| 4.30 |
|
| |
|
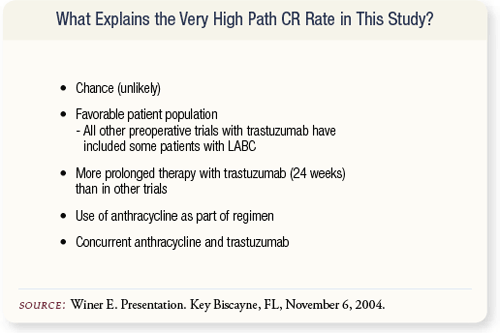
SLIDE 4.30 It’s possible but unlikely that chance alone contributed to the high pathologic CR rate in this study. The trial did not include patients with Stage III disease. Prolonged administration of trastuzumab — 24 weeks of therapy — may also have played an important role. Finally, the concurrent use of an anthracycline and trastuzumab may have led to the high pathologic CR rate.
|
| |
|
|
Select publications
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

