John R Mackey, MD |
EDITED COMMENTS |
 BCIRG-001: Phase III randomized trial comparing adjuvant TAC to FAC BCIRG-001: Phase III randomized trial comparing adjuvant TAC to FAC
Study design
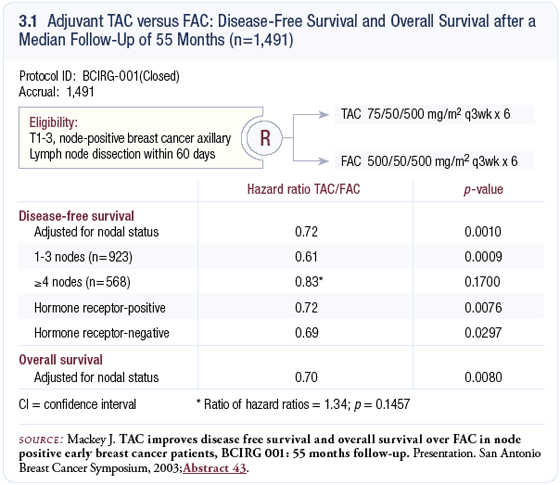
In our first study, BCIRG-001, 1,500 women from 21 countries were randomly assigned to six cycles of adjuvant TAC (docetaxel, doxorubicin and cyclophosphamide) or FAC (5-FU, doxorubicin and cyclophosphamide) (3.1). The women enrolled in the trial had node-positive disease.
Overall results
We now have mature results with five years of follow-up. The trial demonstrated that adjuvant TAC significantly improved disease-free survival by 28 percent in relative terms (p = 0.001). Overall survival was also strikingly improved; the trial demonstrated a 30 percent relative reduction in mortality with adjuvant TAC, which was an absolute six percent improvement in overall survival (3.1).
Benefit according to number of positive axillary lymph nodes
For the final analysis at 590 events, the trial was powered to determine whether a correlation exists between the number of positive axillary lymph nodes and the benefit associated with adjuvant TAC. Investigators evaluated the data to determine whether a significant difference exists in the ratio of the hazard ratios for patients with four or more involved axillary nodes and patients with one to three involved axillary nodes.
The analysis to date shows no correlation. The numbers indicate an independent, significant improvement with TAC in the patients with one to three positive nodes. In the patients with four or more positive nodes, the numbers are favoring TAC with fewer recurrences, but a statistically significant p-value is not reported. According to the protocol, we must compare the hazard ratios for those two groups even though no difference exists between the two (3.1); hence, we are in agreement with the FDA that adjuvant TAC is of benefit to women with node-positive breast cancer, regardless of the number of positive axillary lymph nodes.
Safety
This would be a perfect story if an increase in side effects did not occur. In fact, TAC was associated with a high rate of febrile neutropenia. Approximately 25 percent of the women receiving TAC experienced an episode of febrile neutropenia, which was not unexpected because primary prophylaxis with G-CSF was not allowed (Martin 2003). We now know that if we were to do the study again and administer TAC with G-CSF, we would see a febrile neutropenia rate, on a per-patient basis, of about three to six percent (Martin 2004).
BCIRG-005: Phase III randomized trial comparing adjuvant TAC to AC followed by docetaxel
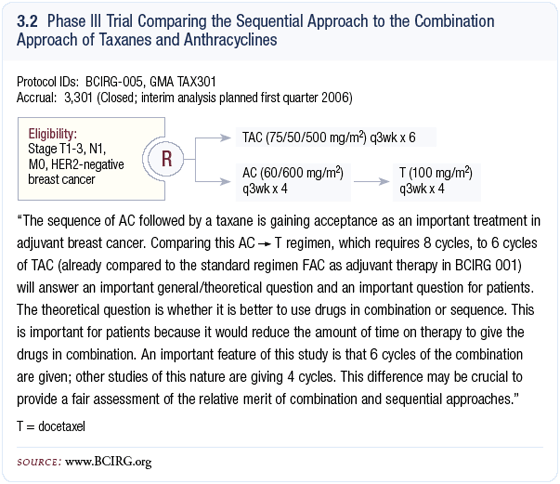
It is questioned whether chemotherapy drugs need to be combined in the adjuvant setting to obtain a maximal effect. Across the spectrum of cancer types, sequential monotherapy is not usually a curative approach. Because paclitaxel could not be safely combined with anthracyclines, sequential therapy became, in some sense, the standard of care; however, a pharmacokinetic interaction does not occur with docetaxel and doxorubicin.
BCIRG-005 is comparing the combination of TAC to AC followed by docetaxel. This study has completed its accrual of over 3,000 patients and we are waiting for mature data to determine whether six cycles of adjuvant TAC is different, in terms of efficacy, from four cycles of AC followed by four cycles of docetaxel.
BCIRG-006: Phase III randomized adjuvant trastuzumab trial
I believe the most exciting adjuvant trial we’ve done in a long time is BCIRG- 006, in which patients with HER2-positive tumors on all of the arms received docetaxel. Patients were randomly assigned to receive: AC followed by docetaxel; AC followed by docetaxel in combination with trastuzumab; or docetaxel, carboplatin and trastuzumab for six cycles with trastuzumab continued for one year.
The trial required the centralized FISH analysis of all of the tumor blocks prior to randomization. All of the 3,200 patients with HER2-positive disease have undergone randomization and treatment. Now we’re in the follow-up phase and are anticipating disease-free survival data by the first quarter of 2006.
BCIRG-006 has an Independent Data Monitoring Committee and an Independent Cardiac Safety Monitoring Committee, which have been meeting regularly to keep a close eye on the cardiac safety data. We’re quite gratified to say that they’re comfortable with what has been happening in the study; they have not requested any amendments to the protocol or any release of data.
I am currently blinded to the cardiac events but, in evaluating the available trial data in aggregate, we are not seeing a substantial cardiac signal. Also, the Independent Cardiac Data Monitoring Committee, which can evaluate the data on a per-arm basis, is not indicating any concern.
Initial selection of adjuvant hormonal therapy in postmenopausal women
At least two thirds of the postmenopausal women we’re seeing have hormone receptor-positive disease. I am on the ATAC Steering Committee and our 47- month data (Baum 2003) and the recently published 68-month data (ATAC Trialists’ 2004) suggest safety and disease-free survival efficacy with anastrozole. The early signals indicate that anastrozole is probably a better drug; however, its potential long-term side effects have to be kept in mind.
When we lay these data on the table, about two thirds of these women choose anastrozole, but one third stay with our old standby, tamoxifen.
Role of aromatase inhibitors in postmenopausal women following two to three years of adjuvant tamoxifen
About 50 percent of my patients are switching to an aromatase inhibitor following two to three years of adjuvant tamoxifen. Even though the disease-free survival data is clean and compelling, women are not switching as frequently as I would have guessed.
Role of aromatase inhibitors in postmenopausal women after five years of adjuvant tamoxifen
Data from the Canadian MA17 trial suggest that after five years of tamoxifen, women receiving extended adjuvant therapy with letrozole do better than women receiving no further therapy (Goss 2003). I’m concerned because that study was closed prematurely.
I’m also disappointed that with 2.5 years of follow-up, we’re not going to have any more meaningful survival data because the trial was unblinded and women were crossed over. I’m a real believer in the importance of overall survival advantages to make definitive treatment recommendations. To date, we have no convincing overall survival data from any of the adjuvant aromatase inhibitor trials.
Although we might switch a woman to letrozole today, we’re not going to have data in the near future to tell us how long we should continue that treatment, nor are we going to have data about the overall survival benefit. Even though it appears to be a safe and effective intervention, I have doubts about the long-term effects, particularly on bone. In addition, I’m disappointed that we don’t have the survival signal or the possibility of obtaining a survival signal from that study.
Select publications
 |
Dr Mackey is a Medical Oncologist at Cross Cancer Institute, Alberta, Canada, Associate Professor of Medical and Experimental Oncology at the University of Alberta, Chair of the Northern Alberta Breast Cancer Program and Canadian Leader of the Breast Cancer International Research Group. |
|

