|

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the cardiac issues with trastuzumab?
DR LOVE: Would you discuss the cardiac issues with trastuzumab?
![]() DR HUNT: The cardiotoxicity documented with trastuzumab, particularly in
the adjuvant trials, may be only the tip of the iceberg in terms of the natural
history of that toxicity.
DR HUNT: The cardiotoxicity documented with trastuzumab, particularly in
the adjuvant trials, may be only the tip of the iceberg in terms of the natural
history of that toxicity.
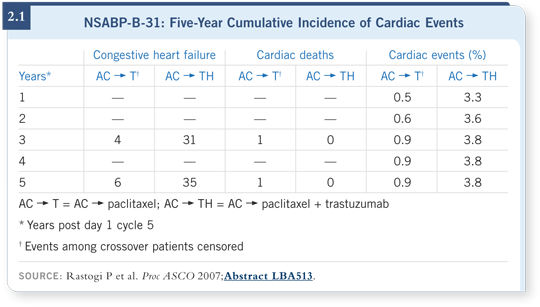
The five-year follow-up of the NSABP-B-31 trial was encouraging in that no burgeoning amount of cardiotoxicity occurred (Rastogi 2007; [2.1]). But doctors taking care of these women for the longer term need to be aware that having been through that course of trastuzumab, in addition to anthracyclines, may place women at higher risk for developing cardiac dysfunction later in life.
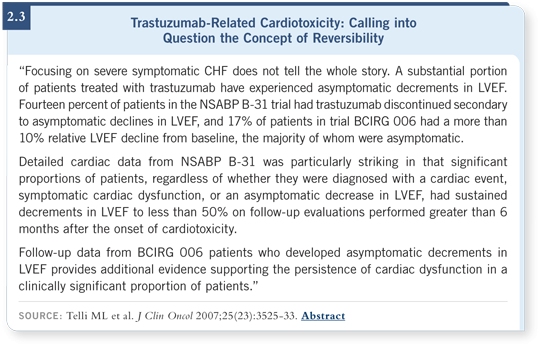
![]() DR LOVE: You were one of the authors on a paper in the Journal of Clinical
Oncology, “Trastuzumab-Related Cardiotoxicity: Calling into Question the Concept of Reversibility” (Telli 2007; [2.3]). In the adjuvant setting with
trastuzumab, how much of the cardiotoxicity is related to anthracycline/trastuzumab and how much to trastuzumab alone?
DR LOVE: You were one of the authors on a paper in the Journal of Clinical
Oncology, “Trastuzumab-Related Cardiotoxicity: Calling into Question the Concept of Reversibility” (Telli 2007; [2.3]). In the adjuvant setting with
trastuzumab, how much of the cardiotoxicity is related to anthracycline/trastuzumab and how much to trastuzumab alone?
![]() DR HUNT: In the data available so far, it appears that trastuzumab without
anthracyclines has a much lower incidence of evident cardiotoxicity than the
combination, at least at the three and five-year follow-ups.
DR HUNT: In the data available so far, it appears that trastuzumab without
anthracyclines has a much lower incidence of evident cardiotoxicity than the
combination, at least at the three and five-year follow-ups.
Whether that will continue to be the case as the natural history of the toxicity plays out, we obviously don’t know. But for now it seems in the trials with the three and five-year follow-up that it’s the combination of the anthracycline and trastuzumab that leads to increased cardiotoxicity.
Track 3
![]() DR LOVE: In your presentation at ASCO 2007, you showed a pie chart
illustrating the reasons behind heart transplantation, and the anthracycline-related cardiomyopathy piece of the pie did not appear to be inconsequential
(Hunt 2007).
DR LOVE: In your presentation at ASCO 2007, you showed a pie chart
illustrating the reasons behind heart transplantation, and the anthracycline-related cardiomyopathy piece of the pie did not appear to be inconsequential
(Hunt 2007).
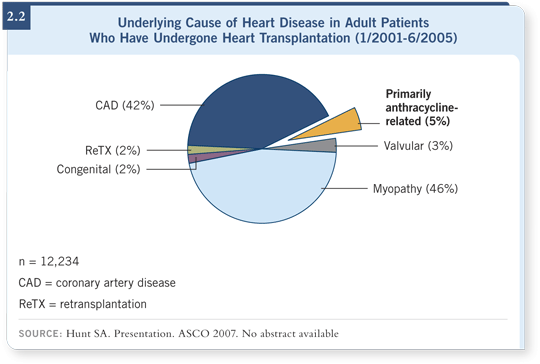
![]() DR HUNT: No, it was not inconsequential, although the absolute numbers
were not large. Every year, the International Society for Heart and Lung
Transplantation publishes data from a registry that has accrued over 100,000
heart transplants over the past 25 years. That pie chart documents the underlying
cause of heart disease that led to the need for transplantation (2.2).
DR HUNT: No, it was not inconsequential, although the absolute numbers
were not large. Every year, the International Society for Heart and Lung
Transplantation publishes data from a registry that has accrued over 100,000
heart transplants over the past 25 years. That pie chart documents the underlying
cause of heart disease that led to the need for transplantation (2.2).
An approximately five percent section in that pie chart is listed as “other.” The majority of that five percent is long-term anthracycline-related cardiotoxicity.
Five percent of 2,500 cases per year in the US is approximately 125 patients. That’s not many patients, but again that’s only the tip of the iceberg. The vast majority of people who have advanced heart disease are not eligible for transplantation for a variety of reasons.
So that 125 is a representative number of patients who are dying of advanced anthracycline-related cardiotoxicities. We may find that a future population — five, 10 or 20 years from today — will have end-stage trastuzumab-related cardiotoxicities. We do not know that, but I believe we should watch for it.
Track 7
![]() DR LOVE: What can be done for patients who are at a higher risk of
developing heart failure, specifically women who’ve received adjuvant
trastuzumab, particularly with an anthracycline? As these women age over
five, 10 or 20 years, how can a medical oncologist, often the primary care
provider, provide optimal cardiovascular support?
DR LOVE: What can be done for patients who are at a higher risk of
developing heart failure, specifically women who’ve received adjuvant
trastuzumab, particularly with an anthracycline? As these women age over
five, 10 or 20 years, how can a medical oncologist, often the primary care
provider, provide optimal cardiovascular support?
![]() DR HUNT: It is important for the primary care physician and the oncologist to
always have in the back of their minds the fact that the patient was exposed to
a potential cardiotoxin, even if it was 20 years ago, and to be aware that any
additional insults can lead to the development of heart failure in later life.
DR HUNT: It is important for the primary care physician and the oncologist to
always have in the back of their minds the fact that the patient was exposed to
a potential cardiotoxin, even if it was 20 years ago, and to be aware that any
additional insults can lead to the development of heart failure in later life.
For instance, Dr Love, if you developed a little bit of hypertension, it might have no effect on you for many years if you didn’t treat it, whereas a woman whose heart has been sensitized by this preexisting damage might be particularly sensitive to even small amounts of hypertension.
![]() DR LOVE: What level of blood pressure would pique your attention in a
patient like this?
DR LOVE: What level of blood pressure would pique your attention in a
patient like this?
![]() DR HUNT: If a woman had undergone potentially cardiotoxic chemotherapy
in the past, I wouldn’t want her blood pressure to be over 120/80 mm Hg.
DR HUNT: If a woman had undergone potentially cardiotoxic chemotherapy
in the past, I wouldn’t want her blood pressure to be over 120/80 mm Hg.
No guidelines on this exist, but with the analogous problem of diabetes, even the national organizations say that someone with diabetes should never have their blood pressure reach over 120 mm Hg.
They would allow you and me to reach 130 mm Hg before initiating therapy, but patients who are so much more prone to developing cardiac dysfunction should be monitored at lower levels.
Track 11
![]() DR LOVE: Can you review the cardiac safety data in the adjuvant
trastuzumab trials?
DR LOVE: Can you review the cardiac safety data in the adjuvant
trastuzumab trials?
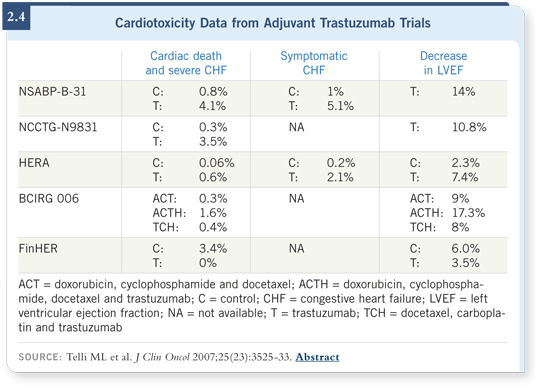
![]() DR HUNT: There were four major trials (NSABP-B-31, NCCTG-N9831,
HERA, BCIRG 006) and a smaller study from Finland (FinHER). The trials
were all different clinical designs, but at the end of the day approximately four
percent of patients at three years had developed overt cardiac toxicity (Telli
2007; [2.4]).
DR HUNT: There were four major trials (NSABP-B-31, NCCTG-N9831,
HERA, BCIRG 006) and a smaller study from Finland (FinHER). The trials
were all different clinical designs, but at the end of the day approximately four
percent of patients at three years had developed overt cardiac toxicity (Telli
2007; [2.4]).
For subclinical cardiac toxicity, such as abnormalities on echocardiography, it was closer to 14 or 15 percent.
Some of those patients with either subclinical or clinical heart failure showed evidence of reversibility. In other words, over time and with treatment, ejection fractions and symptoms improved or totally resolved.
However, a significant fraction did not improve. It is not clear whether or not those conditions that seemed to resolve will stay resolved. That is why longer-term follow-up of these cohorts of women is essential.