|

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Can you summarize where we are currently in terms of cardiac safety and trastuzumab/chemotherapy?
DR LOVE: Can you summarize where we are currently in terms of cardiac safety and trastuzumab/chemotherapy?
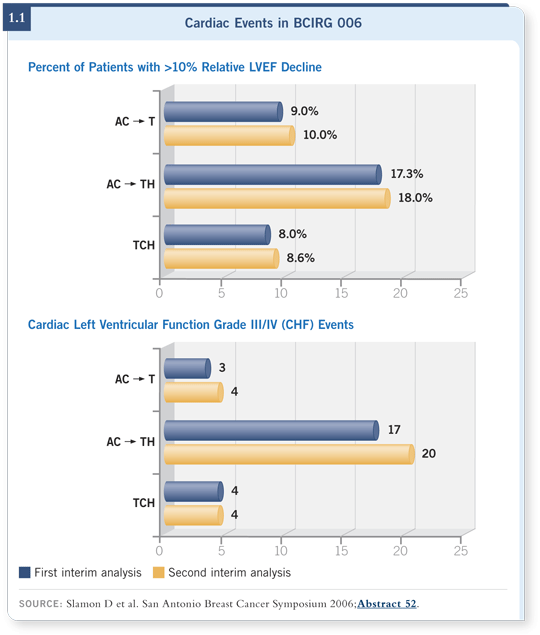
![]() DR SLAMON: The BCIRG 006 trial (Slamon 2006) had a nonanthracycline
arm. The study demonstrated that cardiac dysfunction in patients who received trastuzumab and anthracyclines, even when trastuzumab was administered
after the anthracycline, increased fourfold to fivefold (1.1).
DR SLAMON: The BCIRG 006 trial (Slamon 2006) had a nonanthracycline
arm. The study demonstrated that cardiac dysfunction in patients who received trastuzumab and anthracyclines, even when trastuzumab was administered
after the anthracycline, increased fourfold to fivefold (1.1).
This toxicity is certainly not short and transient, as people have been saying — a few weeks or months — it’s clearly longer. If you factor in what also happens to these patients in the long term as they age — as we cure them, which is what we hope we do — and they accrue other health issues such as hypertension, obesity, diabetes and other cardiac insults, our concern is that the cardiac dysfunction will increase even further. Certainly, some of the SEER database analysis indicates that’s the case with anthracycline therapy (Pinder 2007) — much more so than we previously thought. Among patients receiving trastuzumab, I’m worried that we will see the incidence go even higher.
Track 4
![]() DR LOVE: Can you discuss TOPO II and how it relates to the treatment of HER2-positive disease?
DR LOVE: Can you discuss TOPO II and how it relates to the treatment of HER2-positive disease?
![]() DR SLAMON: Approximately eight percent of the world population of patients with breast cancer have coamplification of HER2 and TOPO II. It turns out that with these patients, you can use an anthracycline alone and obtain a trastuzumab-like benefit without using the trastuzumab, but that’s for a small number of patients.
DR SLAMON: Approximately eight percent of the world population of patients with breast cancer have coamplification of HER2 and TOPO II. It turns out that with these patients, you can use an anthracycline alone and obtain a trastuzumab-like benefit without using the trastuzumab, but that’s for a small number of patients.
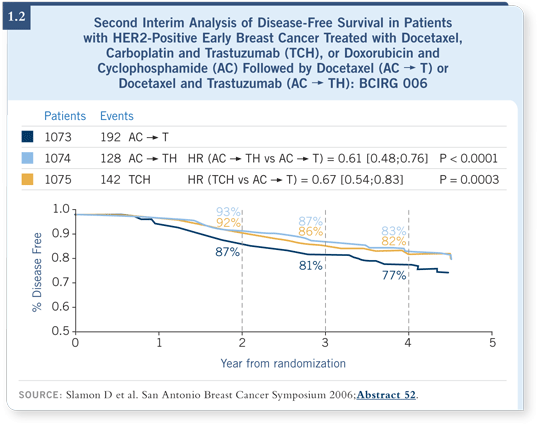
If you use trastuzumab without the anthracycline, you obtain the benefit that’s been reported, and if you use a nonanthracycline chemotherapy, such as TCH, you obtain exactly the same benefit. The curves are identical — they overlap completely (Slamon 2006; [1.2]).
This indicates that 92 percent of women derive no benefit from anthracycline-based therapy in terms of an incremental improvement, but they derive all the toxicity — the cardiomyopathies, congestive heart failure, leukemia and myelodysplasia. So I don’t believe these agents have a role in the adjuvant setting if you can use trastuzumab.
Track 5
![]() DR LOVE: What about the issue of using anthracyclines for patients with HER2-negative tumors?
DR LOVE: What about the issue of using anthracyclines for patients with HER2-negative tumors?
![]() DR SLAMON: The clinical data seemed to indicate no benefit for the patients with HER2-negative disease, but we wanted to investigate a step further. So we asked how often TOPO II was amplified in patients from the BCIRG 005 trial (Press 2007), which included HER2-negative cases as determined by FISH.
DR SLAMON: The clinical data seemed to indicate no benefit for the patients with HER2-negative disease, but we wanted to investigate a step further. So we asked how often TOPO II was amplified in patients from the BCIRG 005 trial (Press 2007), which included HER2-negative cases as determined by FISH.
Out of 1,800 cases analyzed, not a single case of TOPO II amplification was recorded. I believe that explains why the analyses from three decades of trials — randomized trials of more than 7,000 patients, conducted by four or five different groups — all showed no incremental benefit from anthracycline-based therapy compared to nonanthracycline-based therapy for the vast majority of patients.
![]() DR LOVE: So what’s your perspective on all this information?
DR LOVE: So what’s your perspective on all this information?
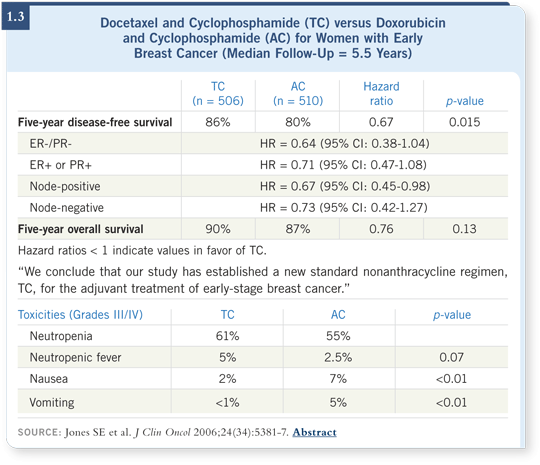
![]() DR SLAMON: We don’t use anthracyclines in the adjuvant setting. The
adjuvant regimen we use for our non-HER2-positive cases, based on the
US Oncology data presented by Steve Jones, is TC (Jones 2006; [1.3]). I’m
always asked if I would use TC even for node-positive disease. The answer is
unequivocally yes. I don’t believe — based on the data, which are significant
and have been around for a while — that an incremental benefit to receiving
anthracyclines occurs for these patients, so we don’t use them.
DR SLAMON: We don’t use anthracyclines in the adjuvant setting. The
adjuvant regimen we use for our non-HER2-positive cases, based on the
US Oncology data presented by Steve Jones, is TC (Jones 2006; [1.3]). I’m
always asked if I would use TC even for node-positive disease. The answer is
unequivocally yes. I don’t believe — based on the data, which are significant
and have been around for a while — that an incremental benefit to receiving
anthracyclines occurs for these patients, so we don’t use them.
Track 8
![]() DR LOVE: One of the biggest areas of controversy continues to be the
management of HER2-positive, node-negative tumors, particularly smaller
than one centimeter. Where are we right now in terms of data related to
that decision?
DR LOVE: One of the biggest areas of controversy continues to be the
management of HER2-positive, node-negative tumors, particularly smaller
than one centimeter. Where are we right now in terms of data related to
that decision?
![]() DR SLAMON: Mike Press and Leslie Bernstein published data on HER2-positive tumors that were smaller than one centimeter (Press 1997; [1.4]). It
turns out that the outcome data for those tumors indicate that those women
have the same outcomes as women with node-positive tumors, so the recurrence
rate at five years, and presumably out further, should be similar.
DR SLAMON: Mike Press and Leslie Bernstein published data on HER2-positive tumors that were smaller than one centimeter (Press 1997; [1.4]). It
turns out that the outcome data for those tumors indicate that those women
have the same outcomes as women with node-positive tumors, so the recurrence
rate at five years, and presumably out further, should be similar.
So the way we treat small tumors that are HER2-positive is to treat them as HER2-positive tumors. The argument we’ve made is that it isn’t so much the size of the tumor or even the number of nodes, it’s how the tumor is wired, and if it’s wired as a HER2-positive tumor, it will behave badly and should be treated with trastuzumab-based therapy.

Track 10
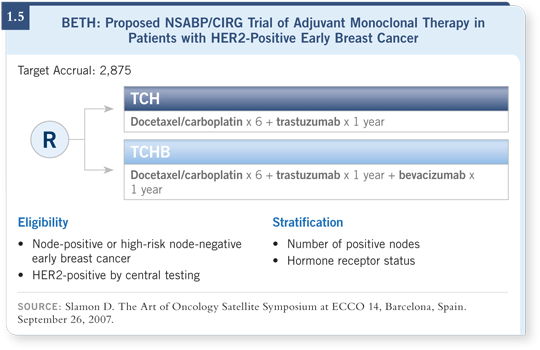
![]() DR LOVE: Can you discuss the BETH trial being launched by the CIRG
and NSABP?
DR LOVE: Can you discuss the BETH trial being launched by the CIRG
and NSABP?
![]() DR SLAMON: We’re evaluating the combination of trastuzumab and
bevacizumab because HER2 upregulates VEGF, which causes tumors to
be more pro-angiogenic. The question is, if you combine bevacizumab and
trastuzumab, what will you observe? Preclinically, remarkable efficacy was
demonstrated — better than either drug alone.
DR SLAMON: We’re evaluating the combination of trastuzumab and
bevacizumab because HER2 upregulates VEGF, which causes tumors to
be more pro-angiogenic. The question is, if you combine bevacizumab and
trastuzumab, what will you observe? Preclinically, remarkable efficacy was
demonstrated — better than either drug alone.
In early clinical trials, in the first-line metastatic disease setting, we used only the two antibodies and achieved response rates of 52 percent and clinical benefits of 83 percent. Moving into the adjuvant setting with the NSABP, this proposed 2,700-patient trial will evaluate TCH with or without bevacizumab (1.5).
![]() DR LOVE: What about the issue of cardiac safety using the combination of
bevacizumab and trastuzumab?
DR LOVE: What about the issue of cardiac safety using the combination of
bevacizumab and trastuzumab?
![]() DR SLAMON: One of the main reasons we built the regimen on a backbone
of docetaxel/carboplatin is that we were worried that any added insult to the
cardiovascular system could be problematic. We know that bevacizumab is
associated with a hypertension rate of approximately 18 percent.
DR SLAMON: One of the main reasons we built the regimen on a backbone
of docetaxel/carboplatin is that we were worried that any added insult to the
cardiovascular system could be problematic. We know that bevacizumab is
associated with a hypertension rate of approximately 18 percent.
As the cardiologists have taught us — and we should have learned from basic cardiac physiology — an increased afterload can be one of the best predictors of inducing left ventricular dysfunction.
We’ll be increasing afterload clinically in approximately one fifth of the patients and perhaps subclinically in an even higher number. We’ll find out what the safety profile shows with TCH and TCHB.